| Numéro |
OCL
Volume 32, 2025
Contaminants in oils and fats / Contaminants des huiles et corps gras
|
|
|---|---|---|
| Numéro d'article | 19 | |
| Nombre de pages | 21 | |
| DOI | https://doi.org/10.1051/ocl/2025014 | |
| Publié en ligne | 17 juin 2025 | |
Review
Mycotoxins in oilseeds and vegetable edible oils: an overview of toxicity, occurrence and exposure☆
Mycotoxines dans les graines oléagineuses et les huiles alimentaires végétales : toxicité, occurrence et exposition
1
TOXALIM (Research Centre in Food Toxicology), Université de Toulouse, INRAE, ENVT, INP-Purpan, UPS, Toulouse, France
2
IRSD, Université de Toulouse, INSERM, INRAE, ENVT, UPS, Toulouse, France
* Corresponding author isabelle.oswald@inrae.fr
Received:
13
February
2025
Accepted:
8
April
2025
Oilseeds and vegetable oils, essential components of the human diet, can be contaminated by various mycotoxins, fungal toxic secondary metabolites. This review presents an overview of (i) the chronic toxicity of mycotoxins, (ii) their occurrence in vegetable oils and oilseeds and (iii) consumer exposure through these foodstuffs. A systematic search was performed to identify reviews, published during the last 10 years, concerning the occurrence of mycotoxins in commonly consumed oils and oilseeds. Around fifteen different mycotoxins were detected, the most common being the regulated mycotoxins: aflatoxins, ochratoxin A, zearalenone, fumonisins and trichothecenes. Emerging mycotoxins such as Alternaria toxins, beauvericin and cyclopiazonic acid were also detected. These toxins have various effects and target multiple organs (kidneys, liver, nervous system, digestive system, etc.) and some of them, such as aflatoxin B1, ochratoxin A and fumonisins, are carcinogenic or possibly carcinogenic to humans. Mycotoxins in oils and oilseeds are sometimes present in quantities exceeding European standards for consumer safety. Although they are consumed in smaller quantities than other food groups (e.g., cereals), the contribution of edible oils to the chronic dietary exposure to various mycotoxins should not be neglected. In the case of certain mycotoxins, such as alternariol monomethyl ether, vegetable oils even account for a significant proportion (around 40%) of average chronic European dietary exposure.
Résumé
Les graines oléagineuses et les huiles végétales, éléments essentiels de l’alimentation humaine, peuvent être contaminées par diverses mycotoxines, métabolites secondaires toxiques produits par des champignons. Cette revue présente (i) une vue d’ensemble de la toxicité des mycotoxines, (ii) de leur présence dans les huiles végétales et les graines oléagineuses et (iii) de l’exposition des consommateurs par le biais de ces denrées alimentaires. Une recherche systématique a été effectuée pour recenser les publications parues au cours des dix dernières années concernant la présence de mycotoxines dans les huiles et les graines oléagineuses couramment consommées. Une quinzaine de mycotoxines différentes ont été identifiées dans les huiles et les graines oléagineuses les plus couramment utilisées, les plus courantes étant les mycotoxines réglementées : aflatoxines, ochratoxine A, zéaralénone, fumonisines et trichothécènes. Des mycotoxines émergentes telles que les toxines d’Alternaria, la beauvéricine et l’acide cyclopiazonique ont également été détectées. Ces toxines ont des effets toxiques variés ciblant plusieurs organes (les reins, le foie, le système nerveux ou le système digestif). L’aflatoxine B1, l’ochratoxine A et les fumonisines, en particulier, sont considérées comme cancérogènes ou possiblement cancérogènes pour l’homme. Les mycotoxines sont parfois présentes dans les huiles et les graines oléagineuses à des niveaux parfois supérieurs aux limites fixées par la réglementation européenne pour la sécurité des consommateurs. Bien qu’elles soient consommées en plus faibles quantités que d’autres aliments tels que les céréales, la contribution des huiles alimentaires à l’exposition alimentaire chronique aux différentes mycotoxines mérite une attention particulière. Dans le cas de certaines mycotoxines, comme l’alternariol monomethyl ether, les huiles végétales représentent même une part significative (environ 40 %) de l’exposition alimentaire chronique moyenne en Europe.
Key words: oils / oilseeds / mycotoxins / toxicity / occurrence
Mots clés : Huiles / graines oléagineuses / mycotoxines / toxicité / occurrence
© A. Durant et al., Published by EDP Sciences, 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Highlights
Oilseeds and vegetable oils are frequently contaminated with regulated (AFB1, OTA, FBs, ZEN and TCTs) or emerging mycotoxins (BEA, Alternaria toxins, CPA) in concentrations sometimes exceeding European standards for consumer safety.
Except for EFSA reports, literature mainly focuses on Asian and African occurrence data.
Mycotoxins identified in oilseeds and vegetable oils have various chronic toxic effects, including carcinogenicity, targeting a wide variety of organs (digestive, renal, nervous systems, etc.).
Although edible oils are not the main contributor to dietary exposure to mycotoxins, their contribution is not negligible and even significant for some mycotoxins like alternariol monomethyl ether.
1 Introduction
Edible oils are important components of the human diet, mainly because of their role in cooking. In addition to their palatability and energy content, their fatty acid content is essential for human health, as they have a major role in neuronal development and cognitive functions, transport fat-soluble vitamins and are involved in the synthesis of steroid hormones and prostaglandins (Tian et al., 2023). A dozen vegetable oils account for 90% of world production, including palm oil, soybean oil, rapeseed oil, sunflower oil, cottonseed oil, peanut oil, olive oil, sesame oil and corn oil (Morin and Pagès-Xatart-Parès, 2012).
Mycotoxins are toxic secondary metabolites produced by filamentous fungi that may be injurious to vertebrates upon ingestion, inhalation, or skin contact (Marin et al., 2013). A large number of mycotoxins have been identified and are produced by various fungal genera, mainly Aspergillus, Penicillium, Alternaria, Fusarium and Claviceps (Bennett and Klich 2003 ; Marin et al., 2013). These molecules are produced under ambient temperature and moisture conditions. Therefore, they can contaminate the whole food chain (with differences from year to year linked to climate conditions), in the field, and during processing, transport and storage (Ostry et al., 2017).
Mycotoxins have diverse chemical structures and exhibit diverse acute and chronic toxic effects on humans and animals, such as carcinogenicity, teratogenicity, mutagenicity, hepatotoxicity, nephrotoxicity, embryotoxicity and immunotoxicity (Karsauliya et al., 2022). On the basis of their individual occurrence and toxicity, seven mycotoxins are regulated in Europe: aflatoxin B1 (AFB1), deoxynivalenol (DON), fumonisin B1 (FB1), ochratoxin A (OTA), patulin (PAT), zearalenone (ZEN) and ergot alkaloids (AEs) (Payros et al., 2021).
Just like the majority of foods and foodstuffs, oilseeds and edible oils can be contaminated by a wide range of contaminants, including mycotoxins (Xia et al., 2021). Oilseeds are particularly susceptible to mycotoxin contamination, as their nutrient-rich composition makes them ideal substrates for fungal growth. Contamination can occur at various stages of production (Zhang et al., 2024). Many mycotoxins are hydrophobic and therefore have an affinity with lipids, so during the extraction of contaminated oilseeds, they may migrate from seeds into extracted oils. (Abdolmaleki et al., 2021). Most of these toxins are heat-stable, so conventional cooking processes, including those concerning oils such as frying, lead to minimal reduction in mycotoxin levels in food (Payros et al., 2021; Liu et al., 2020).
Contamination of oilseeds and edible oils therefore contributes to the global human exposure to mycotoxins and represents a hazard to human health. It also causes considerable losses to the economy of the food industry by reducing agricultural production and commercial revenues (Karsauliya et al., 2022).
This review aims to examine the impact of mycotoxins present in oilseeds and edible vegetable oils on human health, addressing their toxicity, their contamination during the production process, and the risks of exposure for consumers.
2 Human toxicity of mycotoxins
With regard to mycotoxins in oils and fats, acute toxicity from dietary mycotoxins in oils and fats is considered low (cf part 3.). We have therefore chosen to focus solely on their chronic effects.
2.1 Aflatoxins
Aflatoxins (AFs) are the most widely studied mycotoxins. They are produced by Aspergillus section flavi species, in particular A. flavus and A. parasiticus (Cimbalo et al., 2020). A distinction is made between aflatoxins B1 and B2, G1 and G2, and the hydroxylated forms M1 and M2 (found mainly in milk), based on their chemical structure. Among these toxins, AFB1 is the most frequently detected in contaminated foods (Marchese et al., 2018). In Europe, the average exposure to AFB1 ranges from 0.08 to 6.95 ng/kg b.w. per day (minimum lower bound (LB) to maximum upper bound (UB))1, depending on the age group concerned (Schrenk et al., 2020a).
After ingestion, AFB1 is rapidly absorbed into the bloodstream, primarily from the small intestine. It binds to plasma albumin and is transported by the hepatic portal system to the liver, where it is metabolised, and thus activated (Payros et al., 2021). AFB1 is a substrate for the cytochromes P450 (CYP) CYP3A4, CYP3A5 and CYP1A2, which convert it to AFB1-exo-8,9-epoxide (AFBO). This compound is a reactive and unstable molecule that is capable of forming DNA adducts at N7-guanyl sites (Schrenk et al., 2020a).
Chronic exposure to AFB1 has several consequences, the main one being hepatic carcinogenesis. International Agency for Research on Cancer (IARC) has classified AFB1 as “carcinogenic to humans” (group 1), since exposure to this toxin has been associated with an increased risk of hepatocellular carcinoma (Claeys et al., 2020). DNA adducts formed as a result of AFB1 metabolism can cause mutations in genes such as the p53 tumour suppressor gene, promoting tumour cell proliferation and development of hepatocarcinoma (Weng et al., 2017). AFB1 can also generate reactive oxygen species (ROS) that activate mitochondrial pathways including apoptosis. In addition to DNA adducts, AFB1 plays a role in carcinogenesis through oxidative stress (Marchese et al., 2018).
In addition to its carcinogenic effects, AFB1 is teratogenic in animal species, including humans (resulting in bone malformations, visceral anomalies, low birth weight, etc.) (da Silva et al., 2021). Exposure to AFB1 also causes growth impairment and stunting (Watson et al., 2017). AFB1 is also immunotoxic and therefore may compromise the ability of both humans and animals to resist infections (Meissonnier et al., 2008).
2.2 Ochratoxin A
Ochratoxins (OTs) are produced by certain species of Aspergillus and Penicillium fungi. The most widespread mycotoxin among them is OTA (Alshannaq and Yu 2017). In Europe, average exposure to OTA ranges from 0.64 to 17.79 ng/kg b.w. per day (minimum LB to maximum UB), depending on the age group concerned (Schrenk et al., 2020b).
When ingested, OTA is mainly absorbed in the jejunum and enters the bloodstream. Once in systemic circulation, it binds 99.98% to albumin and other serum proteins, which gives it a long half-life in humans (up to 35 days). This toxin is poorly metabolised in vivo and tends to accumulate in tissues, particularly the kidney, which is the major target organ of OTA toxicity (Schrenk et al., 2020b). In the kidney, OTA is recognised by the OAT1 and OAT3 anion transporters from basolateral side (blood) and by OAT4 from apical side (proximal tube). This allows OTA to pass from the blood to the proximal tubules by tubular secretion, and to be reabsorbed at all nephron segments, leading to OTA accumulation in renal tissues (Schrenk et al., 2020b).
Chronic exposure to OTA has toxic effects on various organs, particularly the kidney. Based on animal data, the IARC has classified OTA as “possibly carcinogenic to humans” (group 2B) (Claeys et al., 2020). However, new data suggest that OTA can be genotoxic, directly damaging DNA. The mechanism of this genotoxicity is still debated. One hypothesis is the formation of DNA adducts by biotransformation of OTA into OTA-quinone (Schrenk et al., 2020b). Indeed, the low levels of DNA adducts found are difficult to relate to the genotoxic effects of OTA observed in vivo. Other studies suggest that OTA genotoxicity is a secondary consequence of oxidative stress generation, but there is little evidence of oxidative DNA damage or lipid peroxidation (Payros et al., 2021).
OTA is mainly known for its nephrotoxicity, the mechanisms of which are inhibition of protein synthesis (due to its structural similarity to phenylalanine, it inhibits the activity of phenylalanine hydroxylase), DNA damage, cell cycle arrest and apoptosis (Khoi et al., 2021). But OTA also exhibits hepatotoxic, immunotoxic, neurotoxic and teratogenic effects on animals and humans (Więckowska et al., 2024).
2.3 Fumonisins
Fumonisins are produced mainly by Fusarium fungi, in particular F. verticillioides and F. proliferatum. Among the 28 analogues in the family, the most common and toxic belong to the type B (FB) (Qu et al., 2022). Among these, FB1 is the most common and toxic analogue, FB2 and FB3 are the second and third most common FBs, while FB4 is found in lower concentrations (Li et al., 2023). In Europe, average exposure to FBs ranges from 0.04 to 1.77 μg/kg bw per day (minimum LB to maximum UB), depending on the age group concerned (EFSA 2014a). Toxicological assessments of FBs were carried out mainly with FB1, FB2-4 having similar toxicological profiles and potency (Knutsen et al., 2018).
FBs are poorly absorbed in the gastrointestinal tract after ingestion (around 4% of the ingested dose is absorbed) and rapidly excreted, mainly in the bile (Payros et al., 2021). Absorbed FBs are distributed to all organs, mainly the liver, kidneys and muscles. These molecules are poorly metabolised in mammalian tissues (mainly by hydrolysis of the ester groups, forming N-fatty acyl FBs (Knutsen et al., 2018).
FBs are structurally similar to sphinganine and sphingosine, the substrates of ceramide synthetase. They are therefore competitive inhibitors of this enzyme, which catalyses the condensation of sphinganine with an acyl-CoA to produce ceramide. FBs thus disrupt the biosynthetic pathway of sphingolipids, widely distributed molecules, especially in biomembranes, which regulate numerous signal transduction pathways and critical cellular functions, such as cell proliferation, differentiation, senescence, apoptosis and carcinogenesis (Qu et al., 2022).
Through this mechanism, FBs can have a variety of toxic effects on organisms, including autophagy, apoptosis, neurotoxicity, immunotoxicity, reproductive and carcinogenic effects. FB1 and FB2 have been recognised as “possibly carcinogenic to humans” (group 2B) by IARC, as a correlation between FB1 and FB2 consumption and increased incidence of oesophageal cancer has been established on humans (Chen et al., 2021). FB1 also has high-affinity for the folate transporters and can impair the transfer of folates to the developing foetus, causing embryotoxic and teratogenic effects such as growth retardation, delayed or incomplete organogenesis, malformations and ultimately foetal death, in several species (Lumsangkul et al., 2019).
2.4 Zearalenone
Zearalenone (ZEN) is produced by Fusarium fungi, in particular F. graminearum. In Europe, average exposure to ZEN ranges from 2.4 to 29 ng/kg b.w. per day (minimum LB to maximum UB) for adults and from 24 to 277 ng/kg b.w. per day for children (EFSA, 2011b).
After ingestion and absorption in the proximal intestine, ZEN is metabolised mainly by enterocytes and hepatocytes with two different pathways. The first one is a reduction of ZEN by cytochrome P450 (CYP450) enzymes, producing α-zearalenol (α-ZOL) and β-zearalenol (β-ZOL). The second one involves the conjugation of ZEN and its metabolites with glucuronic acid, catalysed by the enzymes UDP-glucuronosyltransferases (UGTs) (Kowalska et al., 2016).
Zearalenone and its metabolites α-ZOL and β-ZOL have a strong structural similarity to 17β-estradiol. They competitively bind to α and β oestrogen receptors (ER), giving them strong hyper oestrogenic and endocrine-disrupting effects on animals, especially prepubertal pigs, and humans (Rai et al., 2020). The metabolites of ZEN have different oestrogenic potentials, with α-ZOL having the highest (Steinkellner et al., 2019). In humans, ZEN can decrease embryo survival and reduce foetal weight, as well as decrease milk production. ZEN is also known to alter the morphology of uterine tissue, leading to reduced levels of the hormones LH and progesterone in women, while in men it reduces sperm count and viability, and impedes spermatogenesis (Ropejko and Twarużek 2021). ZEN is also toxic to other organs, such as the liver, immune cells and intestine (Payros et al., 2021). It is suspected of promoting the development of breast cancer, as studies demonstrate that it can stimulate the growth of MCF cells (human breast cancer cells with ER receptors) (Rai et al., 2020). ZEN is not classifiable as to its carcinogenicity to humans (group 3) by IARC based on limited evidence of its carcinogenicity in animals (Claeys et al., 2020).
2.5 Trichothecenes
Trichothecenes (TCTs) are one of the main classes of mycotoxins of concern to humans and the food industry. This family comprises more than 200 structurally related compounds, divided into 4 groups (A to D) according to their hydroxyl and acetoxy functional side groups (McCormick et al., 2011). The most common in food are type A TCTs HT-2 toxin (HT2) and T-2 toxin (T2), and type B deoxynivalenol (DON). Type A and B TCTs are produced mainly by several Fusarium species (Terciolo et al., 2018). In Europe, average exposure to T2 and HT2 ranges from 1.82 to 64.8 ng/kg b.w. per day (minimum LB to maximum UB) depending on the age group concerned (Arcella et al., 2017). Chronic dietary exposure to DON was estimated to be on average between 0.2 and 2 μg/kg b.w. per day (minimum LB to maximum UB) depending on the population group (Knutsen et al., 2017a).
T-2 toxin can be rapidly absorbed from the digestive tract after ingestion and metabolised mainly in the liver. The main metabolic pathways observed are hydrolysis, hydroxylation, conjugation, and de-epoxidation, leading to a wide variety of metabolites, including HT-2 toxin (Janik et al., 2021). DON is absorbed in the upper intestine, at a rate of between 52.7 and 100% in the jejunum. DON is metabolised via two different routes: tissue biotransformation via enterohepatic passage (glucuronidation), and by the intestinal microbiota (leading to a detoxified form: de-epoxy-deoxynivalenol) (Payros et al., 2016; Payros et al., 2021).
At a cellular level, the major effect of TCT is related to their ability to bind to the 60S ribosomal subunit and inhibit ribosomal peptidyltransferase activity (Garreau de Loubresse et al., 2014). This leads to a ribotoxic stress response characterized by inflammation and apoptosis but also to an inhibition of RNA and protein synthesis as well as stress of endoplasmic reticulum, oxidative stress, and autophagy (Garofalo et al., 2025). Through these mechanisms, T-2 and HT-2 toxins have cytotoxic effects through apoptosis in various cell types and organs, including brain, gastrointestinal tract, skin, and the immune system, especially on monocytes during their differentiation into dendritic cells or macrophages, thus impacting immune system function (Janik et al., 2021). Neurotoxic effects have also been observed, associated with the ability of T-2 and HT-2 toxins to cross the blood-brain barrier (De Ruyck et al., 2015).
DON exposure is responsible for deleterious effects in the intestine (lesions, reduced number and size of villi, impaired barrier function) following loss of tight junction protein expression and exacerbation of apoptosis in intestinal tissue. A link between DON exposure and chronic inflammatory bowel disease has been established (Payros et al., 2020). The effects of DON on immune system function have been studied in animal models. DON has been shown to exacerbate inflammation by inducing the production of proinflammatory cytokines and the secretion of immunoglobulins (Knutsen et al., 2017a).
TCTs are not directly genotoxic, DON and T-2 toxin are not classifiable as to their carcinogenicity to humans (group 3) by IARC (Meneely et al., 2023) based on limited evidence of their carcinogenicity in animals. Nevertheless, it has been shown that these compounds can promote the genotoxicity of other genotoxic contaminants in the diet (Garofalo et al., 2023).
2.6 Emerging mycotoxins
Besides the above mentioned ?regulated? mycotoxins, there are other toxins that are less documented, not routinely determined, and not regulated; they are called “emerging” mycotoxins (Hasuda and Bracarense, 2024). These include some mycotoxins present in oils and oilseeds, especially, cyclopiazonic acid (CPA) (produced by some Aspergillus and Penicillium fungi species), enniatins (ENs), beauvericin (BEA) (both Fusarium-derived toxins often co-occurring species and often found together in the same foodstuffs), and Alternaria mycotoxins (especially alternariol (AOH), alternariol monomethyl ether (AME), altenuene (ALT), altertoxins (ATXs), tenuazonic acid (TeA) and tentoxin (TEN)).
To date, there is no robust estimate of average chronic exposure to CPA in humans. By contrast, chronic mean exposure to BEA in Europe (minimum LB to maximum UB) ranges from 0.003 to 0.050 µg/kg b.w. per day, and chronic mean exposure to the sum of ENs ranges from 0.42 to 1.82 µg/kg b.w. per day (EFSA, 2014b). In the adult population, the mean chronic dietary exposure to AOH ranges from 1.9 to 39 ng/kg b.w. per day, from 0.8 to 4.7 ng/kg b.w. per day for AME, from 36 to 141 ng/kg b.w. per day for TeA, and from 0.01 to 7 ng/kg b.w. per day for TEN (minimum LB to maximum UB) (EFSA, 2011a).
There are few data on CPA toxicity in humans. CPA has been mainly described as a highly selective inhibitor of Ca2+-ATPase in skeletal, cardiac and smooth muscle sarcoplasmic reticulum (Uyama et al., 1993). This enzyme is involved in muscle contraction and relaxation by regulating intracellular calcium concentration by transporting calcium into the endoplasmic reticulum from the cell cytosol (Gehlert et al., 2015). Little is known about the effects of chronic exposure to CPA on the human body. Studies on mice show that CPA causes neurotoxic effects similar to the side-effects of anti-psychotropic drugs (hypokinesia, catalepsy, etc.) (Nishie et al., 1985).
In vitro, BEA and ENs are known to impair cell viability, block cell cycle, induce apoptosis, and generate ROS in several cell types (Hasuda and Bracarense, 2024). There are few data concerning the in vivo effects of these mycotoxins, the majority of the studies being performed on birds. Their mechanism of action is not fully understood and their metabolites are not characterised yet (EFSA, 2011a). Despite their clear in vitro toxicity, the majority of in vivo data suggest a more limited toxicity. Nevertheless, long-term studies particularly on mammals, are needed to assess the toxicity of chronic exposure to BEA and ENs (Caloni et al., 2020; Gruber-Dorninger et al., 2017).
Alternaria mycotoxins can be categorized into two distinct groups: namely, genotoxic and non-genotoxic mycotoxins. Genotoxic mycotoxins (i.e. AOH, AME, and ATXs) have been shown to induce DNA strand breaks via topoisomerase inhibition and ROS in various cell lines, primarily through their effects on topoisomerases and generation of ROS). Conversely, non-genotoxic mycotoxins such as TeA and TEN mainly cause in vitro cytotoxicity (Louro et al., 2024; Zhang et al., 2025). In vivo hazard characterisation of Alternaria toxins focused mainly on the commercially available AOH, AME and TeA. Limited toxicity data are available for ALT, and TEN yet (Louro et al., 2024). AOH, AME and TeA, are known to induce toxic effects in animals, including fetotoxic and teratogenic effects. (Marin et al., 2013) In vitro studies have suggested that AOH and AME may also have moderate endocrine disruptive effects and disturb the immune response and adaptive immune system (Louro et al., 2024). A correlation between occurrence of Alternaria toxins and an increased incidence of oesophageal cancer has been indicated (Solhaug et al., 2016). However, no indication of carcinogenicity for Alternaria toxins to humans has been listed by IARC yet (Cimbalo et al., 2020). Currently, there is no regulation for the presence of Alternaria mycotoxins in food worldwide. However, it should be noted that the European Union has published guidelines for the maximum concentration of AOH, AME, and TeA in specific food products (Zhang et al., 2025).
Emerging mycotoxins are causing growing concern because of their potential adverse effects on human health, and their regulatory status remains limited. In-depth research and rigorous hazard assessment are needed to better determine their impact on human health.
3 Mycotoxin contamination of oilseeds and edible vegetable oils
Following the discussion on toxicity of the main mycotoxins found in oilseeds and edible oils, the occurrence of these toxins in oilseeds and edible oils will be summarised. A comprehensive literature review of reviews published during the last 10 years (2014-2024) was carried out using Web of Science and PubMed (all databases) with the following keywords: Mycotoxin*, Occurrence, Oil*. Grey literature and non-English articles were not included. 54 initial publications were obtained after this initial search, 13 of which were selected for building Tables 1 and 2. An additional literature search (with the same keywords and exclusion criteria) has been conducted on research articles published between 2023 and 2025. These articles, too recent to be cited in existing reviews, have been included in Tables 1 and 2 in order to keep the data up to date. The detailed selection process is presented in Figure 1.
Oilseeds contamination by mycotoxins (based on literature review).
3.1 Occurrence of mycotoxins in oilseeds
Depending on their growing conditions, oilseeds can be contaminated by mycotoxins at different stages in the agricultural chain (before, during or after harvest, during transport or storage). Available data on the occurrence of mycotoxins in oilseeds mainly concern Asia and Africa.
Mycotoxin contamination of oilseeds varies from region to region, but also from one type of oilseed to another. (Table 1) Five of the most consumed oilseeds in the world (corn, peanuts, sunflower seeds, soybean seeds and olives) are discussed below. Various mycotoxins were identified in the oilseeds analysed. Among these, the most common include mycotoxins that are dangerous to health and subject to regulation (AFs, OTA, FB1 and FB2, ZEN, as well as TCTs, especially DON, T-2 and HT-2 toxins). AFs, particularly AFB1, are notable for their presence in the largest number of samples.
3.1.1 Corn
Corn is widely used around the world to produce oil for the food industry. It is mainly used in processed foods, due to its stability and relatively low cost. Numerous data are available on mycotoxin contamination in corn. Table 1 shows the multiple contamination of corn seeds from Africa, China, South or Central America by various mycotoxins (AFs, OTA, ZEN, FBs, DON and other TCTs). In these developing countries, hot weather, old production techniques and poor storage conditions favours the growth of fungi and the production of mycotoxins (An et al., 2024). Infection of corn by A. flavus is frequent and corn is described as a food staple commonly associated with AFs contamination (de Almeida et al., 2019). Aflatoxin contamination was observed in almost all the reports in Table 1. It is worth noting that with global climate change, concerns are growing about aflatoxin contamination, not just in developing countries, but also in Europe (Battilani et al., 2016, Bailly et al., 2018). Out of 98 samples of maize from Spanish suppliers between 2015 and 2019, 9% were positive for AFB1, with 3% having aflatoxin levels above the European limit of 5 ng/g (Tarazona et al., 2020).
3.1.2 Peanuts
Peanuts are widely grown for the extraction of edible oil and the preparation of peanut butter. They are frequently analysed for the presence of fungi and their mycotoxins, especially AFs (Bhat and Reddy, 2017). AFs were found in peanut samples from several countries in Africa, Brazil, Australia and Sri Lanka. Peanut is also reported to contain high amounts of FBs, OTA, ZEN, and CPA (produced by A. flavus, often co-detected with aflatoxins). (Table 1).
3.1.3 Sunflower seeds
Sunflower seeds can be eaten whole be as a snack, but their main application is the extraction of the oil, highly popular for cooking and frying because of its richness in unsaturated fatty acids (linoleic and oleic acid). Like most oilseeds, sunflower seeds are frequently contaminated by AFs, particularly AFB1 as shown in Tanzania (Ji et al., 2024). However, these seeds are also frequently contaminated by fungi of the genus Alternaria and the mycotoxins they produce, especially TeA as shown in European samples.
3.1.4 Soybean
Soybeans are used for the production of processed food and sauces. Unlike other oilseeds, soybeans are rarely contaminated with AFs. Because of their low zinc availability, they are not a good substrate for Aspergillus fungi (Bordin et al., 2014). Fusarium fungi producing ZEN and DON are most frequently isolated on soybeans as illustrated in Table 1 for South American and African countries (Abia et al., 2013).
3.1.5 Olive
Olives are eaten mainly as an aperitif or as a culinary ingredient, or pressed to extract the oil, which is widely used in the Mediterranean diet. The contamination of olives by mycotoxins is poorly investigated. El Adlouni et al. (2006) studied the presence of OTA in 10 samples of black olives from Morocco and all the samples were contaminated with OTA at levels of 0.2 to 1.2 μg/kg. It should be noted that olive tissues exhibit natural antifungal properties for fungal growth, as olive trees have natural defences against fungi. The leaves and fruit contain flavonoids and phenolic compounds, which have antimicrobial properties (Muzzalupo et al., 2020).
3.1.6 Rapeseed
Rapeseed is grown almost exclusively for oil production (Bhat and Reddy, 2017). Brazauskienė et al. (2006) studied fungal contamination and mycotoxin production in rapeseed originating from Lithuania. According to their study, rapeseed samples showed the presence of fungi of the genus Aspergillus, Cladosporium, Alternaria, Penicillium and Fusarium as dominant fungi. In addition, DON, produced by Fusarium fungi, was detected in various samples at concentrations of between 164 and 183 μg/kg. In two rapeseed varieties, AFs were also detected at levels of between 1 and 3.1 μg/kg (Bhat and Reddy, 2017).
3.1.7 Sesame
Sesame is grown mainly in Africa and Asia for its seeds, which can contain up to 60% oil. Mycotoxin contamination is frequently reported in sesame and sesame-based products (Anyogu et al., 2024). Table 1 shows the multiple contamination of sesame seeds from Africa and Asia by several mycotoxins (AFs, FB1, DON, OTA and BEA).
Fapohunda et al. (2018) collected 24 sesame seed samples from farmers’ stores in Nigeria and detected several regulated mycotoxins, especially DON, FB1 and AFB1 (Anyogu et al., 2024). Pongpraket et al. (2020) studied the occurrence of 16 mycotoxins, including emerging mycotoxins, in 200 samples of white and black sesame marketed in Thailand. In this study, 21.5% of the samples were contaminated by at least one mycotoxin. The mycotoxins most frequently detected in samples of both black and white sesame were AFs, but also the emerging mycotoxin BEA (Pongpraket et al., 2020). BEA and ENs are often detected together but no mention of ENs is made in this paper, suggesting that they have not been investigated.
3.2 Mycotoxin transfer from oilseeds to edible oil during extraction and effects of refining
3.2.1 Oil extraction and refining process
Once they have reached maturity, the oilseeds are harvested. In order to limit mycotoxin contamination, as part of good agricultural practices, the seeds often undergo a selection process to eliminate seeds visibly contaminated by fungi. Seeds are then dried to reduce the moisture content to a safe level for storage in silos, until the oil is extracted.
There are two main extraction methods for edible oils: mechanical pressing and solvent extraction. Mechanical pressing is recommended for oilseeds with high oil content such as flaxseed, sunflower and corn. This method is simpler, safer and requires fewer steps compared with solvent extraction (Bordin et al., 2014). The solvent extraction method is based on the principle that oils are insoluble in water but soluble in certain organic solvents. Industrially, the most widely used solvent for oil extraction is n-hexane. This method is more efficient but generates solvent residues, leading to health, environmental and qualitative issues. Several solvents and more environmentally-friendly extraction techniques (such as supercritical fluids) have been developed to replace hexane extraction, with various limitations (Gagnon et al., 2021). At the end of the extraction stage, the virgin oil is obtained but extraction methods do not eliminate mycotoxins. The mycotoxins present in oilseeds are shared between the oil and the cake (Bordin et al., 2014).
Several factors, especially the extraction method, can influence the transfer of mycotoxins from the oilseeds into oils. The choice of the solvent plays a crucial role in this process. For example, hexane, commonly used for extracting vegetable oil from oilseeds, does not extract AFs, thus limiting the transfer of AFs into the oil. By contrast, alternative solvents, such as ethanol or isopropanol, have been shown to be effective in extracting both oil and AFs, thus facilitating the transfer of mycotoxins into the oil (Bordin et al., 2014).
To improve sensory quality (taste, odour, clarity) nutritional and stability characteristics, nutritional value and stability of edible oils, they may undergo refining steps after extraction (Pages et al., 2010). First, the degumming removes phospholipids, trace metals, and impurities. Then the oil undergoes deacidification by alkaline neutralisation to remove free fatty acids and bleaching to remove pigments that can affect the quality and oxidation stability of the oil. The bleaching stage generally involves high temperature treatment for 20 to 40 minutes using acid-activated bleaching clays and activated carbon. The final stage in the refining process is deodorisation by vacuum steam distillation, which removes the odour as well as volatile compounds and contaminant residues (Wen et al., 2023).
Oil quality significantly influences mycotoxin levels. AFs are relatively insoluble in non-polar solutions, but strong oxidation of oils can increase their polarity, enhancing their solubility (Bordin et al., 2014). Additionally, blending oils from various sources can also affect mycotoxin solubility (Bordin et al., 2014).
3.2.2 Effects of oil refining on mycotoxins
During the multiple stages of oil extraction from oilseeds, some of the mycotoxins initially present migrate to the oil and cake (Ji et al., 2024). Unrefined oils do not undergo heating process or additional chemical treatment and generally contain higher concentrations of mycotoxins, while the physico-chemical conditions used during the successive refining stages partially eliminate the mycotoxins present in the oil (Zio et al., 2020).
Degumming has been shown to partially eliminate mycotoxins from the oil, up to 60% for DON (Guo et al., 2022) and up to 23.90% for ZEN (Ma et al., 2020). The subsequent neutralization step (deacidification under alkali conditions), has been identified as the most effective phase in eliminating mycotoxins from edible oils, achieving up-to 98.94% reduction for AFB1 (Ji et al., 2016), 64.82% for ZEN (Ma et al., 2020), 88.85% for OTA (Lu et al., 2022) and 80% for DON (Guo et al., 2022). During this step, when the oil is treated with alkali (e.g., soda) and centrifuged, a significant proportion of mycotoxins accumulate in the sediment phase. Residual mycotoxins in the supernatant are subsequently transferred to the rinse water (Javanmardi et al., 2022). Increasing the neutralization temperature can improve mycotoxin degradation, from 67.07% to 87.88% reduction for ZEN (Ma et al., 2020). The subsequent bleaching process further reduces the residual mycotoxins levels, efficiently reducing AFs and TCTs, although it is less effective at eliminating ZEN. The final step, deodorisation, performed at high temperatures, has also been shown to reduce concentrations of AFs, ZEN, and TCTs (Kamimura et al., 1986).
Refining processes can induce structural changes in mycotoxins. ZEN, for example, may undergo reversible degradation at low temperatures (Karlovsky et al., 2016; Ma et al., 2020). OTA can be hydrolysed up to 80% during alkali refining to form OP-OTA (a lactone ring-opened derivative) which mainly accumulates in the soap stock, raising concerns about the safety of refining by-products (Lu et al., 2022). DON can also be partly degraded during refining and converted into norDON B (Guo et al., 2022).
3.3 Occurrence of mycotoxins in edible oils
Most of the articles in the literature search concern data on the occurrence of mycotoxins in edible oils produced and marketed outside Europe. However, data on the occurrence of mycotoxins in edible oils marketed in Europe are available in the various EFSA reports.
3.3.1 Edible oils contamination by mycotoxins, based on literature review
A high occurrence of mycotoxins in edible oils is documented in several reviews. As for oilseeds, the most common are AFs, likely due to their lipophilic properties. (Table 2)
Edible oils contamination by mycotoxins (based on literature review)
3.3.2 Corn oil
Notably, no report documented AFB1 contamination in corn oil, whereas a large number of reports found AFs contamination in corn. Corn oil is generally an industrially-produced oil and undergoes a complete refining process, which effectively eliminates the AFs (Kamimura et al., 1986). On the other hand, these processes are less effective in removing ZEN, TCTs and FBs, whose contamination has been demonstrated by various reports mentioned in Table 2.
Escobar et al. (2013) mention the contamination of corn oils by FB1 and DON, rather hydrophilic toxins, which is surprising at first sight since they are very polar and not very soluble in the oil itself. Two hypotheses are suggested in this study. The first is based on prior findings suggesting that in the dry milling process, these mycotoxins may concentrate in the bran and germ fractions intended for oil production. The second hypothesis is that during the process, the water-soluble mycotoxins may be dissolved in the processing water (steep water) or distributed among the by-products of the process.
3.3.3 Peanut oil and butter
Peanut oil accounts for 2.8% of world domestic consumption of vegetable oils for 2023–2024 (USDA, 2025). It is widely used in Asia while peanut butter is popular in Western (particularly in the USA) and African cooking (Shephard, 2018). The reports in Table 2 all indicate contamination by at least one type of AF, with AFB1 being present in the greatest quantities. There is a marked difference in the amount of AFB1 found in different geographical areas.
Sun et al. (2011) and Zhou et al. (2023) detected a relatively low concentration of AFB1 in peanut oils from Chinese economically active areas. In addition to aflatoxins, Zhou et al. (2023) detected OTA as well as the emerging mycotoxins BEA and ENB—frequently co-occurring toxins as they are both produced by Fusarium fungi. In contrast, Elzupir et al. (2010) investigated 21 samples of commercial peanut oil from Khartoum State in Sudan and 100% of the samples were contaminated with AFs with higher levels. These results can be explained by the quality of the peanut raw materials and the traditional oil extraction methods in Sudan. Developed countries generally market refined oils free from significant aflatoxin levels whereas developing countries like Sudan generally consume crude, unrefined artisanal oils, which may contain concerning levels of aflatoxins (Shephard, 2018).
3.3.4 Sunflower oil
Sunflower oil accounts for 9.7% of world domestic consumption of vegetable oils in 2023/24 (USDA, 2025). Junsai et al. (2021) investigated mycotoxin contamination in 50 samples of sunflower oil distributed to markets in Thailand. AFB1 and AFG1 were found to contaminate sunflower oil samples, but with the lowest percentage of mycotoxin contaminations compared to the other types of oils. No details are given of any refining of these oil samples. Mohammed, Munissi et al. (2018) measured the level of AFs in 40 samples of sunflower seeds and 21 samples of unrefined sunflower oil in Tanzania. They observed a low occurrence of AFs in the oil compared with the seeds, which coincides with the data in Tables 1 and 2. AFs appear to exhibit low solubility in sunflower oil during extraction. Zhou et al. (2023) investigated 24 sunflower oils marketed in China, reporting contamination by both regulated (ZEN, T-2 toxin and OTA) and an emerging mycotoxin (AME).
3.3.5 Soybean oil
Soybean oil accounts for 28.1% of world domestic consumption of vegetable oils in 2023/24 (USDA, 2025). The reports mentioned in Table 2 report low AFs concentrations in soybean oils (Ji et al., 2024). On the other hand, Junsai et al. (2021) detected ZEN at an average concentration of 59.31 μg/kg in 50 samples of soybean oil in Thailand.
3.3.6 Olive oil
Olive oil account for 1.1% of world domestic consumption of vegetable oils in 2023/24 (USDA, 2025). It is generally produced in southern Europe (e.g., Greece, Spain, Italy) as well as in Turkey and Morocco. The olive tree is an emblematic plant of the Mediterranean, and olive oil occupies an important place in Mediterranean culinary cultures. Also boosted by its nutritional composition and health benefits, it is increasingly popular in the rest of the world (Palma and Padilla, 2012). The reports presented in Table 2 show contamination of olive oil samples by several mycotoxins. Due to limited storage capacity, traditional oil producers are often obliged to store olives for several months before pressing, leading to fungal colonisation of the fruit. In Morocco, olives harvested in November are sometimes delayed until May. This could explain variation in mycotoxin contamination between olives (Table 1) and olive oils (Table 2) (Tantaoui-Elaraki et al., 2018).
Junsai et al. (2021) investigated mycotoxin contamination in 50 samples of olive oil distributed to markets in Thailand. AFs, ZEN and FBs were the mycotoxins found most frequently. The emerging mycotoxin BEA was also detected. BEA and ENs are often detected together but no mention of ENs is made in this paper, suggesting that they have not been investigated. Zhou et al. (2023) studied 85 olive oils marketed in China, primarily originating from Southern Europe, and detected AOH and AME, two other emerging mycotoxins.
3.3.7 Rapeseed oil
Rapeseed oil account for 15.8% of world domestic consumption of vegetable oils in 2023/24 (USDA, 2025). It is widely used around the world, especially in Europe, both as a table oil and in the processed food industry, due to its relatively low cost (Fénart, 2004). The residues obtained after the oil extraction process are also sometimes used as animal feed, as they are believed to be highly nutritious (Bhat and Reddy, 2017).
The reports presented in Table 2 show contamination of rapeseed oil by ZEN. ZEN is produced by Fusarium fungi, which have been reported to contaminate rapeseed (Bhat and Reddy, 2017). However, DON, also produced by Fusarium fungi and reported in the seeds, was not found in the oil.
3.3.8 Sesame oil
Contamination of sesame oil by AFs has been reported in four reports in Table 2. Elzupir et al., 2010 investigated 14 samples of commercial sesame oil from Khartoum State in Sudan. All sesame oil samples were contaminated with AFs. It should be noted however, that in Sudan, like peanuts oils, sesame oils are often extracted artisanally with small-scale presses from low-grade seeds and suboptimal storage conditions (Elzupir et al., 2010). Zhou et al. (2023) investigated 21 sesame oils marketed in China and showed contamination by both regulated (ZEN and AFB1) and emerging mycotoxins (AME, BEA and ENB).
3.3.9 Palm oil
Palm oil is extracted from the pulp of palm fruits, of which the mycotoxin contamination remains poorly characterised. The fruit is not widely consumed, but palm oil accounts for 34.6% of world domestic consumption of vegetable oils in 2023/24 (USDA, 2025). Junsai et al. (2021) investigated mycotoxin contamination in 50 samples of palm oil distributed to markets in Thailand. AFB1, AFB2, AFG2 and OTA were found in contaminated palm oil samples. Nevertheless, mycotoxin concentrations in these samples were relatively low and within permissible EU safety limits.
3.3.10 Occurrence of mycotoxins in oilseeds and edible oils in Europe, based on EFSA opinions
As already mentioned, most of the data available in the existing literature concerns foodstuffs from countries outside Europe. To compensate for this geographical bias, values supplied by the european member states of EFSA have been included.
The scarcity of published data for Europe could be explained by concentrations too low to report, making so-called ‘negative’ data more difficult to publish. Due to climatic conditions and agricultural practices that favor fungal growth, some foodstuffs might be more susceptible to contamination (Nji et al., 2022). In Europe, good farming practices, plant health measures, and advanced storage techniques are commonly implemented to mitigate the risks of contamination. Regulatory frameworks such as those supported by EFSA play a key role in collecting and analysing data on food safety, including mycotoxin contamination, which helps guide science-based policies.
The data from the various EFSA opinions on the concentration of certain mycotoxins (AFs, OTA, ZEN, T-2 and HT-2 toxins, and Alternaria toxins) in oilseeds and oils marketed in Europe are summarised in Table 3.
OTA and AFB1 concentrations in oilseeds and edible oils from Europe are generally comparatively lower than global averages, potentially due to differences in climate, fungal contamination and /or in regulations. (Table 2) Indeed, mycotoxins are strictly regulated in Europe and these low values are to be expected.
On the other hand, average concentrations for T-2, HT-2 and ZEN are fairly comparable in Europe and the rest of the world. However, for ZEN, most of the samples correspond to maize oils, which show three times higher contamination than the other oils analysed (EFSA, 2011b). This high contamination has already been mentioned in several previous studies and is explained by the fact that maize provides a favourable substrate for the development and spore production of Fusarium species, especially F. culmorum and F. graminearum, two ZEN-producing species (Gimeno et al., 2020).
Average concentrations of Alternaria toxins are significantly higher in Europe than in the rest of the world, particularly for TeA, which has an average concentration of up to 300.8 μg/kg (UB) (Arcella et al., 2016). These are emerging toxins, the prevalence of which has not yet been studied to any great extent compared with regulated toxins. This difference can therefore be attributed to more advanced detection methods.
Occurrence of mycotoxins in oilseeds and edible oils in Europe reported by EFSA
3.4 Human exposure to mycotoxins through consumption of vegetable oils
The contribution of edible oils to mycotoxin exposure depends not only on their mycotoxin contamination, but also on the quantity and types of oil consumed, and therefore on eating habits. In Europe, this contribution has been studied by EFSA in its risk assessments for various regulated and emerging mycotoxins. (Table 4)
Contribution of edible oils to the chronic dietary exposure to mycotoxins across EFSA risk assessment reports At the LB, results below the limit of quantification (LOQ) or limit of detection (LOD) were replaced by zero; at the upper bound (UB), the results below the LOD were replaced by the value of the LOD and those below the LOQ were replaced by the value reported as LOQ.
3.4.1 Aflatoxin B1
Edible fats and oils contribute significantly of the mean chronic dietary exposure of European adults and children to AFB1, up to 40% (LB) for high consumers of peanut butter. However, as AFB1 is carcinogenic, it is strictly regulated in Europe and overall exposure to this mycotoxin is negligible.
3.4.2 Ochratoxin A
The contribution of oilseeds and vegetable oils to the average chronic dietary exposure of European adults to OTA is very low (less than 1% (UB and LB)). The main contributors to OTA exposure are coffee, cereal-based products, fruit, vegetables and meat products (meat and processed meats).
3.4.3 Zearalenone
While vegetable oils are not the main contributor to the average chronic exposure of European adults to ZEN, they still account for a significant proportion, between 5 (UB) and 9% (LB) of the overall exposure.
3.4.4 T-2 and HT-2 toxins
Consumption of edible oils differs significantly across regions, including within Europe, reflecting various cultural and economic factors. These variations influence the contribution of vegetable oils to chronic dietary exposure to mycotoxins. For example, sunflower oil is particularly prevalent in Eastern European countries such as Hungary and Romania while the olive oil consumption is widely consumed across Mediterranean countries. It has especially been established that sunflower oil accounts for approximately 26% (LB) of the average chronic dietary exposure of European adults to T2 and HT-2 toxins. Age is also a key factor influencing variations in mycotoxin exposure, as eating habits and nutritional requirements differ between age groups. Olive oil, for example, accounts for a more significant proportion of mycotoxin exposure in elderly people (around 4% in LB).
3.4.5 Alternaria toxins
Vegetable oils account for a relatively small proportion of the average chronic dietary exposure of European adults to Alternaria toxins (between 1 and 6% for AOH, TeA and TEN). On the other hand, vegetable oils are one of the main contributors to average chronic dietary exposure to AME, accounting for 40% (LB).
In most cases, consumer exposure does not exceed a few per cent of chronic dietary exposure, but some instances reveal elevated exposure levels, particularly for T2 and HT-2 toxins, AFB1 and AME. On the other hand, overall exposure is relatively low, particularly for AFB1, which is strictly regulated. Consumption of vegetable oils does not therefore appear to be a major risk factor in consumer exposure to mycotoxins, but they should not be neglected when assessing this exposure.
4 Conclusion
Although the contribution of vegetable edible oils to overall dietary exposure to mycotoxins is relatively small compared with other food like cereals, their contamination represents a growing concern for food safety worldwide. This article highlights the contamination of the main edible oils consumed worldwide by various mycotoxins, both regulated (such as AFB1, OTA, etc.) and emerging (such as Alternaria toxins or BEA). This is particularly true for virgin oils, although while refining processes can reduce the concentration of certain mycotoxins, they do not always eliminate them completely. These mycotoxins have various toxic effects, affecting different organs (liver, kidneys, nervous system, immune system or reproductive system), exposing consumers to significant health risks. Moreover, they are capable of interacting synergistically or with other food contaminants, sometimes increasing their toxic effects (Alassane-Kpembi et al., 2017; Garofalo et al., 2023; Willoquet et al., 2024). This is a key challenge in mycotoxin risk assessment because in Europe, regulations are based on the individual toxicity of mycotoxins, but foodstuffs are very often contaminated by several mycotoxins at the same time (Streit et al., 2012). The lack of knowledge about their combined effects of these co-occurring mycotoxins represents a major gap that needs to be addressed (Alassane-Kpembi et al., 2017; Khoshal et al., 2019). Another major challenge is climate change, which has many consequences, especially in temperatures and precipitation, intensifying extreme events and leading to alternating periods of prolonged drought and excessive rainfall. (Duchenne-Moutien and Neetoo 2021). These factors affect plant growth, infection by mycotoxinogenic fungi (directly or not) and modifies the distribution of mycotoxins around the world (Zingales et al., 2022; Casu et al., 2024). For example, AFs are produced by Aspergillus spp., which are thermotolerant fungal species, adapted to warmer climates. As a consequence, AFs generally contaminate crops cultivated in tropical/subtropical climates; with the spate of droughts and high temperatures in Europe, an increased aflatoxin contamination has already been observed in Europe, raising long-term food safety concerns (Casu et al., 2024; Bailly et al., 2025).
This review highlights the need for further research in understanding the factors influencing mycotoxin contamination in oilseeds and oils. A global analysis of the occurrence of mycotoxins in oilseeds and oils, incorporating data from all continents, is imperative to offer a comprehensive global overview. The development of predictive models that can anticipate shifts in mycotoxin prevalence due to climate change is also necessary. Furthermore, it would be useful to improve analytical sensitivity for mycotoxin detection for trace-level and emerging mycotoxin detection in diverse oil matrices. Last but not least, robust mitigation strategies are essential to reduce mycotoxin contamination in oilseeds and oils. In terms of risk assessment, it is imperative to consider the cumulative effects of mycotoxin mixtures, as well as the potential for edible oils to be contaminated with other toxicants.
Funding
This work was supported by INRAE-INSERM Exposome et santé (Uritox Project).
Conflicts of interest
The authors declare that they have no conflicts of interest in this article.
Author contribution statement
Writing − original draft and visualisation : Aurore Durant; Writing − Review & Editing: Aurore Durant, Philippe Pinton, Olivier Puel, Isabelle P. Oswald; Project administration and Supervision: Isabelle P. Oswald.
References
- Abdallah FM, Girgin G and Baydar T. 2019. « Mycotoxin Detection in Maize, Commercial Feed, and Raw Dairy Milk Samples from Assiut City, Egypt ». Vet Sci. 6: 57. https://doi.org/10.3390/vetsci6020057. [CrossRef] [Google Scholar]
- Abdolmaleki K, Khedri S, Alizadeh L, et al. 2021. « The mycotoxins in edible oils: An overview of prevalence, concentration, toxicity, detection and decontamination techniques ». Trends in Food Sci Technol. 115: 500–511. https://doi.org/10.1016/j.tifs.2021.06.057. [Google Scholar]
- Abia W, Warth B, Sulyok M, et al. 2013. « Determination of multi-mycotoxin occurrence in cereals, nuts and their products in Cameroon by liquid chromatography tandem mass spectrometry (LC-MS/MS) ». Food Control 31: 438–53. https://doi.org/10.1016/j.foodcont.2012.10.006. [CrossRef] [Google Scholar]
- Ait Mimoune N. 2017. Study of contamination by Aspergillus species Aspergillus section Flavi and by aflatoxins of some dried fruits dried fruits used in confectionery. PhD thesis in Biological Sciences. ENS Kouba, Alger, Algeria [Google Scholar]
- Aït Mimoune N, Riba A, Verheecke C, et al. 2016. « Fungal contamination and mycotoxin production, by Aspergillus spp. in nuts and sesame seeds ». J Microbiol biotechnol Food Sci. 5: 301–5. https://doi.org/10.15414/jmbfs.2016.5.4.301-305. [CrossRef] [Google Scholar]
- Alassane-Kpembi I, Schatzmayr G, Taranu I, et al. 2017. « Mycotoxins Co-Contamination: Methodological Aspects and Biological Relevance of Combined Toxicity Studies ». Crit Rev Food Sci Nutr. 57: 3489–3507. https://doi.org/10.1080/10408398.2016.1140632. [CrossRef] [PubMed] [Google Scholar]
- Alshannaq A, and Yu JH. 2017. « Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food ». Int J Environ Res Public Health. 14: 632. https://doi.org/10.3390/ijerph14060632. [CrossRef] [Google Scholar]
- An N, Shang N, Zhao X, et al. 2024. « Occurrence, Regulation, and Emerging Detoxification Techniques of Aflatoxins in Maize: A Review ». Food Rev Int. 40: 92–114. https://doi.org/10.1080/87559129.2022.2158339. [CrossRef] [Google Scholar]
- Anyogu A, Somorin YM, Oladipo AO, et al. 2024. « Food safety issues associated with sesame seed value chains: Current status and future perspectives ». Heliyon. 10: e36347. https://doi.org/10.1016/j.heliyon.2024.e36347. [CrossRef] [Google Scholar]
- Arcella D, Eskola M and Gómez RJA. 2016. « Dietary Exposure Assessment to Alternaria Toxins in the European Population ». EFSA J. 14: e04654. https://doi.org/10.2903/j.efsa.2016.4654. [Google Scholar]
- Arcella D, Gergelova P, Innocenti ML, et al. 2017. « Human and Animal Dietary Exposure to T-2 and HT-2 Toxin ». EFSA J. 15: e04972. https://doi.org/10.2903/j.efsa.2017.4972. [Google Scholar]
- Bailly S, Mahgubi AE, Carvajal-Campos A, et al. 2018. « Occurrence and Identification of Aspergillus Section Flavi in the Context of the Emergence of Aflatoxins in French Maize ». Toxins 10: 525. https://doi.org/10.3390/toxins10120525. [CrossRef] [PubMed] [Google Scholar]
- Bailly S, ElMahgubi A, Puel O, et al. 2025. « Implantation of Aspergillus Section Flavi in French Maize and Consequences on Aflatoxin Contamination of Maize at Harvest: Three-Year Survey ». Toxins. 17: 155. https://doi.org/10.3390/toxins17040155. [CrossRef] [Google Scholar]
- Battilani P, Toscano P, Van der Fels-Klerx HJ, et al. 2016. « Aflatoxin B1 Contamination in Maize in Europe Increases Due to Climate Change ». Sci Rep. 6: 24328. https://doi.org/10.1038/srep24328. [CrossRef] [Google Scholar]
- Bennett JW and Klich M. 2003. « Mycotoxins ». Clin Microbiol Rev. 16: 497–516. https://doi.org/10.1128/cmr.16.3.497-516.2003 [Google Scholar]
- Bhat R and Reddy KRN. 2017. « Challenges and issues concerning mycotoxins contamination in oil seeds and their edible oils: Updates from last decade ». Food Chem. 215: 425–37. https://doi.org/10.1016/j.foodchem.2016.07.161. [CrossRef] [Google Scholar]
- Bordin K, Sawada MM, da Costa Rodrigues CE, et al. 2014. « Incidence of Aflatoxins in Oil Seeds and Possible Transfer to Oil: A Review ». Food Eng Rev. 6: 20–28. https://doi.org/10.1007/s12393-014-9076-9. [Google Scholar]
- Brazauskienė I, Petraitienė E and Mankevičienė A. 2006. « Effects of genotype and environmental factors on rapeseed contamination with mycotoxins and mycotoxin-producing fungi ». Ekologija. 3: 14–20. [Google Scholar]
- Bumbangi NF, Muma J.B, Choongo K, et al. 2016. « Occurrence and factors associated with aflatoxin contamination of raw peanuts from Lusaka district’s markets, Zambia ». Food Control 68: 291–96. https://doi.org/10.1016/j.foodcont.2016.04.004. [CrossRef] [Google Scholar]
- Caloni F, Fossati P, Anadón A, et al. 2020. « Beauvericin: The beauty and the beast ». Environ Toxicol Pharmacol. 75: 103349. https://doi.org/10.1016/j.etap.2020.103349. [CrossRef] [Google Scholar]
- Casu A, Camardo Leggieri M, Toscano P et al. 2024. « Changing climate, shifting mycotoxins: A comprehensive review of climate change impact on mycotoxin contamination ». Compr Rev Food Sci Food. 23: 13323. https://doi.org/10.1111/1541-4337.13323. [CrossRef] [Google Scholar]
- Chauhan YS, Wright GC, Rachaputi RCN, et al. 2010. « Application of a Model to Assess Aflatoxin Risk in Peanuts ». J Agric Sci. 148: 341–51. https://doi.org/10.1017/S002185961000002X. [CrossRef] [Google Scholar]
- Chen J, Wei Z, Wang Y, et al. 2021. « Fumonisin B1: Mechanisms of toxicity and biological detoxification progress in animals ». Food Chem Toxicol. 149: 111977. https://doi.org/10.1016/j.fct.2021.111977. [Google Scholar]
- Chiotta ML, Fumero MV, Cendoya E, et al. 2020. « Toxigenic fungal species and natural occurrence of mycotoxins in crops harvested in Argentina ». Rev Argent Microbiol. 52: 339–47. https://doi.org/10.1016/j.ram.2020.06.002. [Google Scholar]
- Chun HS, Ok HE, Kim HJ, et al. 2006. « Risk Assessment of Aflatoxins in Food Products Consumed in South Korea ». In 13th World Congress of Food Science & Technology, 458. Nantes, France: EDP Sciences. https://doi.org/10.1051/IUFoST:20060458. [Google Scholar]
- Cimbalo, A, Alonso-Garrido M, Font G, et al. 2020. « Toxicity of mycotoxins in vivo on vertebrate organisms: A review ». Food Chem Toxicol. 137: 111161. https://doi.org/10.1016/j.fct.2020.111161. [CrossRef] [Google Scholar]
- Claeys L, Romano C, De Ruyck K, et al. 2020. « Mycotoxin Exposure and Human Cancer Risk: A Systematic Review of Epidemiological Studies ». Compr Rev Food Sci Food Saf. 19: 1449–64. https://doi.org/10.1111/1541-4337.12567. [CrossRef] [Google Scholar]
- De Almeida L, Williams R, Soares DM. et al. 2019. « Aflatoxin levels in maize and peanut and blood in women and children: The case of Timor-Leste ». Sci Rep. 9: 13158. https://doi.org/10.1038/s41598-019-49584-1. [CrossRef] [Google Scholar]
- De Ruyck K, De Boevre M, Huybrechts I, et al. 2015. « Dietary mycotoxins, co-exposure, and carcinogenesis in humans: Short review ». Mutat Res Rev Mutat Res. 766: 32–41. https://doi.org/10.1016/j.mrrev.2015.07.003. [CrossRef] [Google Scholar]
- Diaz GJ, Krska R and Sulyok M. 2015. « Mycotoxins and cyanogenic glycosides in staple foods of three indigenous people of the Colombian Amazon ». Food Addit Contam Part B Surveill. 8: 291–97. https://doi.org/10.1080/19393210.2015.1089948. [Google Scholar]
- Dissanayake DMMP and Manage PM. 2009. « Aflatoxin contamination in peanuts commercially available in Sri Lanka ». Vidyodaya J of Sci 14:151–159. [Google Scholar]
- Dmmp D and Manage PM. 2010. « Aflatoxin Contamination in Peanuts Commercially Available in Sri Lanka ». Vidyodaya J Sci. 14. https://vjs.sljol.info/articles/1540. [Google Scholar]
- Duchenne-Moutien R.A. and Neetoo H. 2021. « Climate Change and Emerging Food Safety Issues: A Review ». J Food Prot 84: 1884–97. https://doi.org/10.4315/JFP-21-141. [CrossRef] [PubMed] [Google Scholar]
- Echodu R, Malinga GM, Kaducu JM, et al. 2019. « Prevalence of aflatoxin, ochratoxin and deoxynivalenol in cereal grains in northern Uganda: Implication for food safety and health ». Toxicol Rep. 6: 1012–17. https://doi.org/10.1016/j.toxrep.2019.09.002. [CrossRef] [Google Scholar]
- EFSA. 2011a. « Scientific Opinion on the Risks for Animal and Public Health Related to the Presence of Alternaria Toxins in Feed and Food ». EFSA J. 9: 2407. https://doi.org/10.2903/j.efsa.2011.2407. [CrossRef] [Google Scholar]
- EFSA. 2011b. « Scientific Opinion on the Risks for Public Health Related to the Presence of Zearalenone in Food ». EFSA J. 9: 2197. https://doi.org/10.2903/j.efsa.2011.2197. [CrossRef] [Google Scholar]
- EFSA. 2014a. « Scientific Opinion on the Risks for Human and Animal Health Related to the Presence of Modified Forms of Certain Mycotoxins in Food and Feed ». EFSA J. 12: 3916. https://doi.org/10.2903/j.efsa.2014.3916. [Google Scholar]
- EFSA. 2014b. « Scientific Opinion on the Risks to Human and Animal Health Related to the Presence of Beauvericin and Enniatins in Food and Feed ». EFSA J. 12: 3802. https://doi.org/10.2903/j.efsa.2014.3802. [Google Scholar]
- El Adlouni C, Tozlovanu M, Naman F, et al. 2006. « Preliminary Data on the Presence of Mycotoxins (Ochratoxin A, Citrinin and Aflatoxin B1) in Black Table Olives “Greek Style” of Moroccan Origin ». Mol Nutr Food Res. 50: 507‑12. https://doi.org/10.1002/mnfr.200600055. [Google Scholar]
- Elzupir AO, Suliman MA, Ibrahim IA, et al. 2010. « Aflatoxins Levels in Vegetable Oils in Khartoum State, Sudan ». Mycotoxin Res. 26: 69–73. https://doi.org/10.1007/s12550-010-0041-z. [CrossRef] [PubMed] [Google Scholar]
- Esan AO, Fapohunda SO, Ezekiel CN, et al. 2020. « Distribution of Fungi and Their Toxic Metabolites in Melon and Sesame Seeds Marketed in Two Major Producing States in Nigeria ». Mycotoxin Res. 36: 361–69. https://doi.org/10.1007/s12550-020-00400-0. [CrossRef] [PubMed] [Google Scholar]
- Escobar J, Lorán S, Giménez I, et al. 2013. « Occurrence and exposure assessment of Fusarium mycotoxins in maize germ, refined corn oil and margarine ». Food Chem Toxicol. 62: 514–20. https://doi.org/10.1016/j.fct.2013.09.020. [CrossRef] [Google Scholar]
- Eskola M, Kos G, Elliott CT, et al. 2020. « Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25% ». Crit Rev Food Sci Nutr. 60: 2773–89. https://doi.org/10.1080/10408398.2019.1658570. [CrossRef] [PubMed] [Google Scholar]
- Fapohunda SO, Anjorin TS, Sulyok M, et al. 2018. « Profile of Major and Emerging Mycotoxins in Sesame and Soybean Grains in the Federal Capital Territory, Abuja, Nigeria ». Euro. J. of Biologic. Res. 8: 121–30. http://dx.doi.org/10.5281/zenodo.1307184 [Google Scholar]
- Fénart E. 2004. « Rapeseed oil and food consumption ». OCL Oilseeds Fats Crops Lipids. 11: 150–152. https://doi.org/10.1051/ocl.2004.0150. [Google Scholar]
- Gagnon Y, Mhemdi H, Delbecq F, et al. 2021. « Extended surfactants and their tailored applications for vegetable oils extraction: an overview ». OCL Oilseeds Fats Crops Lipids. 28: 7. https://doi.org/10.1051/ocl/2020062. [Google Scholar]
- Gao Z, Luo K, Zhu Q, et al. 2023. « The natural occurrence, toxicity mechanisms and management strategies of Fumonisin B1: A review ». Environ Pollut. 320: 121065. https://doi.org/10.1016/j.envpol.2023.121065. [CrossRef] [Google Scholar]
- Garofalo M, Payros D, Penary M, et al. 2023. « A Novel Toxic Effect of Foodborne Trichothecenes: The Exacerbation of Genotoxicity ». Environ Pollut. 317: 120625. https://doi.org/10.1016/j.envpol.2022.120625. [CrossRef] [Google Scholar]
- Garofalo M, Payros D, Taieb F, et al. 2025. « From ribosome to ribotoxins: understanding the toxicity of deoxynivalenol and Shiga toxin, two food borne toxins ». Crit Rev Food Sci Nutr. 65: 193–205. https://doi.org/10.1080/10408398.2023.2271101. [CrossRef] [PubMed] [Google Scholar]
- Garreau de Loubresse N, Prokhorova I, Holtkamp W, et al. 2014. « Structural basis for the inhibition of the eukaryotic ribosome ». Nature. 513: 517–22. https://doi.org/10.1038/nature13737. [CrossRef] [PubMed] [Google Scholar]
- Gehlert S, Bloch W, and Suhr F. 2015. « Ca2+-Dependent Regulations and Signaling in Skeletal Muscle: From Electro-Mechanical Coupling to Adaptation ». Int J Mol Sci. 16: 1066–95. https://doi.org/10.3390/ijms16011066. [CrossRef] [Google Scholar]
- Gimeno A, Kägi A, Drakopoulos D, et al. 2020. « From laboratory to the field: biological control of Fusarium graminearum on infected maize crop residues ». J Appl Microbiol. 129: 680–694. https://doi.org/10.1111/jam.14634. [Google Scholar]
- Granados-Chinchilla F, Molina A, Chavarría G, et al. 2017. « Aflatoxins occurrence through the food chain in Costa Rica: Applying the One Health approach to mycotoxin surveillance ». Food Control 82: 217–26. https://doi.org/10.1016/j.foodcont.2017.06.023. [CrossRef] [Google Scholar]
- Gruber-Dorninger C, Novak B, Nagl V, et al. 2017. « Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants ». J Agric Food Chem. 65: 7052–7070. https://doi.org/10.1021/acs.jafc.6b03413. [CrossRef] [PubMed] [Google Scholar]
- Guo Y, Lu T, Shi J, et al. 2020. « Identification of Deoxynivalenol and Degradation Products during Maize Germ Oil Refining Process ». Foods. 11: 1720. https://doi.org/10.3390/foods11121720. [Google Scholar]
- Hanvi MD, Lawson-Evi P, Bouka EC, et al. 2021. « Aflatoxins in maize dough and dietary exposure in rural populations of Togo ». Food Control 121: 107673. https://doi.org/10.1016/j.foodcont.2020.107673. [CrossRef] [Google Scholar]
- Hasuda AL and Bracarense APFRL. 2024. « Toxicity of the emerging mycotoxins beauvericin and enniatins: A mini-review ». Toxicon 239: 107534. https://doi.org/10.1016/j.toxicon.2023.107534. [CrossRef] [PubMed] [Google Scholar]
- He Z, Chen Z, Mo Y, et al. 2023. « Assessment of the Adverse Health Effects of Aflatoxin Exposure from Unpackaged Peanut Oil in Guangdong, China ». Toxins 15: 646. https://doi.org/10.3390/toxins15110646. [CrossRef] [PubMed] [Google Scholar]
- Hidalgo-Ruiz JL, Romero-González R, Martínez Vidal JL, et al. 2019. « A rapid method for the determination of mycotoxins in edible vegetable oils by ultra-high performance liquid chromatography-tandem mass spectrometry ». Food Chem. 288: 22–28. https://doi.org/10.1016/j.foodchem.2019.03.003. [CrossRef] [Google Scholar]
- Hove M, De Boevre M, Lachat C, et al. 2016. « Occurrence and risk assessment of mycotoxins in subsistence farmed maize from Zimbabwe ». Food Control 69: 36–44. https://doi.org/10.1016/j.foodcont.2016.04.038. [CrossRef] [Google Scholar]
- Hudu A.R, Addy F, Mahunu G.K, et al. 2024. « Zearalenone Contamination in Maize, Its Associated Producing Fungi, Control Strategies, and Legislation in Sub-Saharan Africa ». Food Sci Nutr. 12: 4489–4512. https://doi.org/10.1002/fsn3.4125. [CrossRef] [Google Scholar]
- Janik E, Niemcewicz M, Podogrocki M, et al. 2021. « T-2 Toxin—The Most Toxic Trichothecene Mycotoxin: Metabolism, Toxicity, and Decontamination Strategies ». Molecules 26: 6868. https://doi.org/10.3390/molecules26226868. [CrossRef] [PubMed] [Google Scholar]
- Javanmardi F, Khodaei D, Sheidaei Z, et al. 2022. « Decontamination of Aflatoxins in Edible Oils: A Comprehensive Review ». Food Rev Int. 38: 1410–26. https://doi.org/10.1080/87559129.2020.1812635. [CrossRef] [Google Scholar]
- Ji J, Wang D, Wang Y, et al. 2024. « Relevant Mycotoxins in Oil Crops, Vegetable Oils, de-Oiled Cake and Meals: Occurrence, Control, and Recent Advances in Elimination ». Mycotoxin Res. 40: 45–70. https://doi.org/10.1007/s12550-023-00512-3. [CrossRef] [PubMed] [Google Scholar]
- Ji N, Diao E, Li X, et al. 2016. « Detoxification and safety evaluation of aflatoxin B1 in peanut oil using alkali refining ». J Sci Food Agric. 96: 4009–4014. https://doi.org/10.1002/jsfa.7592. [Google Scholar]
- Junsai T, Poapolathep S, Sutjarit S, et al. 2021. « Determination of Multiple Mycotoxins and Their Natural Occurrence in Edible Vegetable Oils Using Liquid Chromatography-Tandem Mass Spectrometry ». Foods 10: 2795. https://doi.org/10.3390/foods10112795. [CrossRef] [PubMed] [Google Scholar]
- Kamala A, Ortiz J, Kimanya M, et al. 2015. « Multiple mycotoxin co-occurrence in maize grown in three agro-ecological zones of Tanzania ». Food Control 54: 208–15. https://doi.org/10.1016/j.foodcont.2015.02.002. [CrossRef] [Google Scholar]
- Kamimura H, Nishijima M, Tabata S, et al. 1986. « Survey of Mycotoxins Contamination in Edible Oil and Fate of Mycotoxins During Oil-Refining Processes ». Food Hyg Saf Sci. 27: 59–63. https://doi.org/10.3358/shokueishi.27.59. [CrossRef] [Google Scholar]
- Karlovsky P, Suman M, Berthiller F, et al. 2016. « Impact of food processing and detoxification treatments on mycotoxin contamination ». Mycotoxin Res. 32: 179–205. https://doi.org/10.1007/s12550-016-0257-7. [Google Scholar]
- Karsauliya K, Yahavi C, Pandey A, et al. 2022. « Co-occurrence of mycotoxins: A review on bioanalytical methods for simultaneous analysis in human biological samples, mixture toxicity and risk assessment strategies ». Toxicon 218: 25–39. https://doi.org/10.1016/j.toxicon.2022.08.016. [CrossRef] [PubMed] [Google Scholar]
- Karunarathna NB, Chandima JF, Munasinghe DMS, et al. 2019. « Occurrence of aflatoxins in edible vegetable oils in Sri Lanka ». Food Control 101: 97–103. https://doi.org/10.1016/j.foodcont.2019.02.017. [CrossRef] [Google Scholar]
- Khoi CS, Chen JH, Lin TY, et al. 2021. « Ochratoxin A-Induced Nephrotoxicity: Up-to-Date Evidence ». Intl J of Mol Sci. 22 (20): 11237. https://doi.org/10.3390/ijms222011237. [CrossRef] [Google Scholar]
- Khoshal AK, Novak B, Martin PGP, et al. 2019. « Co-occurrence of DON and Emerging Mycotoxins in Worldwide Finished Pig Feed and Their Combined Toxicity in Intestinal Cells ». Toxins. 11: 727. https://doi.org/10.3390/toxins11120727. [CrossRef] [Google Scholar]
- Kiani AK, Medori MC, Bonetti G, et al. 2022. « Modern Vision of the Mediterranean Diet ». J Prev Med Hyg 63: E36–E36. https://doi.org/10.15167/2421-4248/jpmh2022.63.2S3.2745. [PubMed] [Google Scholar]
- Knutsen HK, Alexander J, Barregård L, et al. 2017a. « Risks to Human and Animal Health Related to the Presence of Deoxynivalenol and Its Acetylated and Modified Forms in Food and Feed ». EFSA J. 15: e4718. https://doi.org/10.2903/j.efsa.2017.4718. [Google Scholar]
- Knutsen HK, Barregård L, Bignami M, et al. 2017b. « Appropriateness to Set a Group Health Based Guidance Value for T2 and HT2 Toxin and Its Modified Forms ». EFSA J. 15: e04655. https://doi.org/10.2903/j.efsa.2017.4655. [Google Scholar]
- Knutsen HK, Barregård L, Bignami M, et al. 2018. « Appropriateness to Set a Group Health-Based Guidance Value for Fumonisins and Their Modified Forms ». EFSA J. 16: e05172. https://doi.org/10.2903/j.efsa.2018.5172. [Google Scholar]
- Kortei NK, Annan T, Akonor PT, et al. 2021. « The Occurrence of Aflatoxins and Human Health Risk Estimations in Randomly Obtained Maize from Some Markets in Ghana ». Sci Rep. 11: 4295. https://doi.org/10.1038/s41598-021-83751-7. [CrossRef] [Google Scholar]
- Kowalska K, Habrowska-Górczyńska DE and Piastowska-Ciesielska AW. 2016. « Zearalenone as an endocrine disruptor in humans ». Environ Toxicol Pharmacol. 48: 141–49. https://doi.org/10.1016/j.etap.2016.10.015. [CrossRef] [Google Scholar]
- Kumar P, Gupta A, Mahato DK, et al. 2022. « Aflatoxins in Cereals and Cereal-Based Products: Occurrence, Toxicity, Impact on Human Health, and Their Detoxification and Management Strategies ». Toxins 14: 687. https://doi.org/10.3390/toxins14100687. [CrossRef] [PubMed] [Google Scholar]
- Lee HS, Nguyen-Viet H, Lindahl J, et al. 2017. « A Survey of Aflatoxin B1 in Maize and Awareness of Aflatoxins in Vietnam ». World Mycotoxin J. 10: 195–202. https://doi.org/10.3920/WMJ2016.2144. [CrossRef] [Google Scholar]
- Li FQ, Li YW, Wang YR, et al. 2009. « Natural Occurrence of Aflatoxins in Chinese Peanut Butter and Sesame Paste ». J Agric Food Chem 57: 3519–24. https://doi.org/10.1021/jf804055n. [CrossRef] [PubMed] [Google Scholar]
- Li S, Yuan P, Fu PY, et al. 2017. « Investigation of contamination situation of fungal toxin in some food in Henan Province from 2014 to 2015 ». China Health Industry 14: 144–147. https://doi.org/10.16659/j.cnki.1672-5654. [Google Scholar]
- Li T, Li J, Wang J, et al. 2023. « The occurrence and management of fumonisin contamination across the food production and supply chains ». J Adv Res. 60: 13‑26. https://doi.org/10.1016/j.jare.2023.08.001. [Google Scholar]
- Liu Y, Jiang Y, Li R, et al. 2017. « Natural occurrence of fumonisins B1 and B2 in maize from eight provinces of China in 2014 ». Food Addit Contam Part B Surveill. 10: 113–17. https://doi.org/10.1080/19393210.2017.1280541. [Google Scholar]
- Liu Y, Galani-Yamdeu JH, Gong YY, et al. 2020. « A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods ». Compr Rev Food Sci Food Saf. 19: 1521–1560. https://doi.org/10.1111/1541-4337.12562 [CrossRef] [Google Scholar]
- Louro H, Vettorazzi A, López de Cerain A, et al. 2024. « Hazard Characterization of Alternaria Toxins to Identify Data Gaps and Improve Risk Assessment for Human Health ». Arch Toxicol. 98: 425–69. https://doi.org/10.1007/s00204-023-03636-8. [CrossRef] [PubMed] [Google Scholar]
- Lu T, Guo Y, Shi J, et al. 2022. « Identification and Safety Evaluation of Ochratoxin A Transformation Product in Rapeseed Oil Refining Process ». J Agric Food Chem. 70: 14931–14939. https://doi.org/10.1021/acs.jafc.2c04532. [CrossRef] [PubMed] [Google Scholar]
- Lu Z, Zhang R, Wu P, et al. 2025. « Occurrence and Exposure Assessment of Zearalenone in the Zhejiang Province, China ». Toxins 17: 9. https://doi.org/10.3390/toxins17010009. [Google Scholar]
- Lumsangkul C, Chiang HI, Lo NW, et al. 2019. « Developmental Toxicity of Mycotoxin Fumonisin B1 in Animal Embryogenesis: An Overview ». Toxins 11: 114. https://doi.org/10.3390/toxins11020114. [CrossRef] [PubMed] [Google Scholar]
- Ma CG, Wang YD, Huang WF, et al. 2020. « Molecular reaction mechanism for elimination of zearalenone during simulated alkali neutralization process of corn oil ». Food Chem. 307: 125546. https://doi.org/10.1016/j.foodchem.2019.125546. [Google Scholar]
- Ma JJ, Shao B, Lin XH, et al. 2011. « Study on the natural occurrence of multi-mycotoxin in cereal and cereal-based product samples collected from parts of China in 2010 ». Chin J Food Hyg. 6: 481–488. [Google Scholar]
- Mahato DK, Lee KE, Kamle M, et al. 2019. « Aflatoxins in Food and Feed: An Overview on Prevalence, Detection and Control Strategies ». Front Microbiol. 10. https://doi.org/10.3389/fmicb.2019.02266. [CrossRef] [Google Scholar]
- Majerus P, Graf N and Krämer M. 2009. « Rapid determination of zearalenone in edible oils by HPLC with fluorescence detection ». Mycotoxin Res. 25: 117–21. https://doi.org/10.1007/s12550-009-0018-y. [Google Scholar]
- Manizan A.L, Oplatowska-Stachowiak M, Piro-Metayer I, et al. 2018. « Multi-mycotoxin determination in rice, maize and peanut products most consumed in Côte d’Ivoire by UHPLC-MS/MS ». Food Control 87: 22–30. https://doi.org/10.1016/j.foodcont.2017.11.032. [CrossRef] [Google Scholar]
- Marchese S, Polo A, Ariano A, et al. 2018. « Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development ». Toxins 10: 214. https://doi.org/10.3390/toxins10060214. [CrossRef] [PubMed] [Google Scholar]
- Marin S, Ramos AJ, Cano-Sancho G, et al. 2013. « Mycotoxins: Occurrence, toxicology, and exposure assessment ». Food Chem Toxicol. 60: 218–37. https://doi.org/10.1016/j.fct.2013.07.047. [CrossRef] [Google Scholar]
- Matumba L, Van Poucke C, Monjerezi M, et al. 2015. « Concentrating aflatoxins on the domestic market through groundnut export: A focus on Malawian groundnut value and supply chain ». Food Control 51: 236–39. https://doi.org/10.1016/j.foodcont.2014.11.035. [CrossRef] [Google Scholar]
- McCormick SP, Stanley AM, Stover NA, et al. 2011. « Trichothecenes: from simple to complex mycotoxins ». Toxins. 3: 802–814. https://doi.org/10.3390/toxins3070802. [Google Scholar]
- Meissonnier GM, Pinton P, Laffitte J, et al. 2008. « Immunotoxicity of aflatoxin B1: impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression ». Toxicol Appl Pharmacol. 231: 142–9. https://doi.org/10.1016/j.taap.2008.04.004. [CrossRef] [Google Scholar]
- Meneely J, Greer B, Kolawole O, et al. 2023. « T-2 and HT-2 Toxins: Toxicity, Occurrence and Analysis: A Review ». Toxins 15: 481. https://doi.org/10.3390/toxins15080481. [CrossRef] [PubMed] [Google Scholar]
- Mmongoyo JA, Wu F, Linz JE, et al. 2017. « Aflatoxin Levels in Sunflower Seeds and Cakes Collected from Micro- and Small-Scale Sunflower Oil Processors in Tanzania ». PLoS One 12: e0175801. https://doi.org/10.1371/journal.pone.0175801. [CrossRef] [PubMed] [Google Scholar]
- Mohammed S, Munissi JJE, and Nyandoro SS. 2018. « Aflatoxins in sunflower seeds and unrefined sunflower oils from Singida, Tanzania ». Food Addit Contam Part B Surveill. 11: 161–66. https://doi.org/10.1080/19393210.2018.1443519. [Google Scholar]
- Morin O and Pagès-Xatart-Parès X. 2012. « Huiles et corps gras végétaux: ressources fonctionnelles et intérêt nutritionnel ». OCL Oils Fats Crops Li. 19: 63–75. https://doi.org/10.1051/ocl.2012.0446. [Google Scholar]
- Mudili V, Siddaih CN, Nagesh M, et al. 2014. « Mould Incidence and Mycotoxin Contamination in Freshly Harvested Maize Kernels Originated from India ». J Sci Food Agric. 94: 2674–83. https://doi.org/10.1002/jsfa.6608. [CrossRef] [Google Scholar]
- Murashiki TC, Chidewe C, Benhura MA, et al. 2017. « Levels and daily intake estimates of aflatoxin B1 and fumonisin B1 in maize consumed by rural households in Shamva and Makoni districts of Zimbabwe ». Food Control 72: 105–9. https://doi.org/10.1016/j.foodcont.2016.07.040. [CrossRef] [Google Scholar]
- Muzzalupo I, Badolati G, Chiappetta A, et al. 2020. « In Vitro Antifungal Activity of Olive (Olea Europaea) Leaf Extracts Loaded in Chitosan Nanoparticles ». Front Bioeng Biotechnol. 8: 151. https://doi.org/10.3389/fbioe.2020.00151. [CrossRef] [Google Scholar]
- Nji QN, Babalola OO, Ekwomadu TI, et al. 2022. « Six Main Contributing Factors to High Levels of Mycotoxin Contamination in African Foods ». Toxins 14: 318. https://doi.org/10.3390/toxins14050318. [CrossRef] [PubMed] [Google Scholar]
- Nishie K, Cole RJ and Dorner JW. 1985. « Toxicity and neuropharmacology of cyclopiazonic acid ». Food Chem Toxicol. 23: 831‑39. https://doi.org/10.1016/0278-6915(85)90284-4. [CrossRef] [Google Scholar]
- Oliveira CAF, Gonçalves NB, Rosim RE, et al. 2009. « Determination of Aflatoxins in Peanut Products in the Northeast Region of São Paulo, Brazil ». Int J Mol Sci. 10: 174–83. https://doi.org/10.3390/ijms10010174. [CrossRef] [Google Scholar]
- Ostry V, Malir F, Toman J, et al. 2017. « Mycotoxins as Human Carcinogens—the IARC Monographs Classification ». Mycotoxin Res. 33: 65–73. https://doi.org/10.1007/s12550-016-0265-7. [CrossRef] [PubMed] [Google Scholar]
- Pages X, Morin O, Birot C, et al. 2010. « Raffinage des huiles et des corps gras et élimination des contaminants ». OCL Oilseeds Fats Crops Lipids. 17: 86–99. https://doi.org/10.1051/ocl.2010.0302. [Google Scholar]
- Palma G and Padilla M. 2012. « Un produit emblématique à la dérive des continents et des consommateurs: l’huile d’olive ». OCL Oilseeds Fats Crops Lipids. 19: 283–9 https://doi.org/10.1051/ocl.2012.0467. [Google Scholar]
- Pandey AK, Samota MK, Kumar A, et al. 2023. « Fungal Mycotoxins in Food Commodities: Present Status and Future Concerns ». Front Sustain Food Syst. 7: 1162595. https://doi.org/10.3389/fsufs.2023.1162595. [CrossRef] [Google Scholar]
- Payros D, Alassane-Kpembi I, Pierron A, et al. 2016. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch Toxicol. 90: 2931–2957. https://doi.org/10.1007/s00204-016-1826-4. [Google Scholar]
- Payros D, Ménard S, Laffitte J, et al. 2020. The food contaminant, deoxynivalenol, modulates the Thelper/Treg balance and increases inflammatory bowel diseases. Arch Toxicol. 94: 3173–3184. https://doi.org/10.1007/s00204-020-02817-z. [Google Scholar]
- Payros D, Garofalo M, Pierron A, et al. 2021. « Les mycotoxines en alimentation humaine: un défi pour la recherche ». Cah Nutr Diet. 56: 170. https://doi.org/10.1016/j.cnd.2021.02.001. [CrossRef] [Google Scholar]
- Pongpraket M, Poapolathep A, Wongpanit K, et al. 2020. « Exposure Assessment of Multiple Mycotoxins in Black and White Sesame Seeds Consumed in Thailand ». J of Food Protec. 83: 1198–1207. https://doi.org/10.4315/JFP-19-597. [CrossRef] [Google Scholar]
- Qian M, Zhang H, Wu L, et al. 2015. « Simultaneous determination of zearalenone and its derivatives in edible vegetable oil by gel permeation chromatography and gas chromatography-triple quadrupole mass spectrometry ». Food Chem. 166: 23–28. https://doi.org/10.1016/j.foodchem.2014.05.133. [CrossRef] [Google Scholar]
- Qu L, Wang L, Ji H, et al. 2022. « Toxic Mechanism and Biological Detoxification of Fumonisins ». Toxins 14: 182. https://doi.org/10.3390/toxins14030182. [CrossRef] [PubMed] [Google Scholar]
- Rai A, Das M and Tripathi A. 2020. « Occurrence and toxicity of a fusarium mycotoxin, zearalenone ». Crit Rev Food Sci Nutr. 60: 2710–29. https://doi.org/10.1080/10408398.2019.1655388. [CrossRef] [PubMed] [Google Scholar]
- Reddy KRN, Farhana NI, and Salleh B. 2011. « Occurrence of Aspergillus spp. and Aflatoxin B1 in Malaysian Foods Used for Human Consumption ». J. of Food Sci. 76: T99–104. https://doi.org/10.1111/j.1750-3841.2011.02133.x. [Google Scholar]
- Ropejko K and Twarużek M. 2021. « Zearalenone and Its Metabolites—General Overview, Occurrence, and Toxicity ». Toxins 13: 35. https://doi.org/10.3390/toxins13010035. [CrossRef] [PubMed] [Google Scholar]
- Sangare-Tigori B, Moukha S, Kouadio HJ, et al. 2006. « Co-occurrence of aflatoxin B1, fumonisin B1, ochratoxin A and zearalenone in cereals and peanuts from Côte d’Ivoire ». Food Addit Contam. 23: 1000–1007. https://doi.org/10.1080/02652030500415686. [CrossRef] [PubMed] [Google Scholar]
- Schrenk D, Bignami M, Bodin L, et al. 2020a. « Risk Assessment of Aflatoxins in Food ». EFSA J. 18: e06040. https://doi.org/10.2903/j.efsa.2020.6040. [Google Scholar]
- Schrenk D, Bodin L, Chipman JK, et al. 2020b. « Risk Assessment of Ochratoxin A in Food ». EFSA J. 18: e06113. https://doi.org/10.2903/j.efsa.2020.6113. [Google Scholar]
- Shephard GS. 2018. « Aflatoxins in peanut oil: food safety concerns ». World Mycotoxin J. 11: 149–58. https://doi.org/10.3920/WMJ2017.2279. [CrossRef] [Google Scholar]
- Silva da JVB, de Oliveira CAF and Ramalho LNZ. 2021. « Effects of Prenatal Exposure to Aflatoxin B1: A Review ». Molecules 26: 7312. https://doi.org/10.3390/molecules26237312. [CrossRef] [PubMed] [Google Scholar]
- Solhaug A, Eriksen GS and Holme JA. 2016. « Mechanisms of Action and Toxicity of the Mycotoxin Alternariol: A Review ». Basic Clin Pharmacol Toxicol. 119: 533–39. https://doi.org/10.1111/bcpt.12635. [CrossRef] [PubMed] [Google Scholar]
- Steinkellner H, Binaglia M, Dall’Asta C, et al. 2019. « Combined hazard assessment of mycotoxins and their modified forms applying relative potency factors: Zearalenone and T2/HT2 toxin ». Food Chem Toxicol. 131: 110599. https://doi.org/10.1016/j.fct.2019.110599. [CrossRef] [Google Scholar]
- Streit E, Naehrer K, Rodrigues I, et al. 2012. « Mycotoxin occurrence in feed and feed raw materials worldwide: long-term analysis with special focus on Europe and Asia ». J Sci Food Agric. 93: 2892–9. https://doi.org/10.1002/jsfa.6225. [Google Scholar]
- Sun G, Wang S, Hu X, et al. 2011. « Co-Contamination of Aflatoxin B1 and Fumonisin B1 in Food and Human Dietary Exposure in Three Areas of China ». Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 28: 461–70. https://doi.org/10.1080/19440049.2010.544678. [CrossRef] [PubMed] [Google Scholar]
- Sun XD, Su P and Shan H. 2017. « Mycotoxin Contamination of Maize in China ». Compr Rev Food Sci Food Saf. 16: 835–49. https://doi.org/10.1111/1541-4337.12286. [CrossRef] [Google Scholar]
- Tantaoui-Elaraki A, Riba A, Oueslati S, et al. 2018. « Toxigenic fungi and mycotoxin occurrence and prevention in food and feed in northern Africa − a review ». World Mycotoxin J. 11: 385–400. https://doi.org/10.3920/WMJ2017.2290. [CrossRef] [Google Scholar]
- Tarazona A, Gómez JV, Mateo F, et al. 2020. « Study on mycotoxin contamination of maize kernels in Spain ». Food Control 118: 107370. https://doi.org/10.1016/j.foodcont.2020.107370. [CrossRef] [Google Scholar]
- Terciolo C, Maresca M, Pinton P, et al. 2018. Review article: Role of satiety hormones in anorexia induction by Trichothecene mycotoxins. Food Chem Toxicol. 121: 701–714. https://doi.org/10.1016/j.fct.2018.09.034. [CrossRef] [Google Scholar]
- Tian M, Bai Y, Tian H, et al. 2023. « The Chemical Composition and Health-Promoting Benefits of Vegetable Oils—A Review ». Molecules 28: 6393. https://doi.org/10.3390/molecules28176393. [CrossRef] [PubMed] [Google Scholar]
- Tölgyesi A, Kozma L and Sharma V.K. 2020. « Determination of Alternaria Toxins in Sunflower Oil by Liquid Chromatography Isotope Dilution Tandem Mass Spectrometry ». Molecules 25: 1685. https://doi.org/10.3390/molecules25071685. [CrossRef] [PubMed] [Google Scholar]
- USDA (United States Department of Agriculture) Foreign Agricultural Service. 2025. Oilseeds: World Markets and Trade. Available online: https://apps.fas.usda.gov/psdonline/circulars/oilseeds.pdf [Google Scholar]
- Uyama Y, Imaizumi Y and Watanabe M. 1993. « Cyclopiazonic acid, an inhibitor of Ca(2+)-ATPase in sarcoplasmic reticulum, increases excitability in ileal smooth muscle. » Br J Pharmacol. 110: 565–72. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2175900/. [CrossRef] [PubMed] [Google Scholar]
- Vasseghian Y, Moradi M, Dragoi EN, et al. 2022. « A review on mycotoxins detection techniques in edible oils ». Int J Environ Anal Chem. 102: 2125–39. https://doi.org/10.1080/03067319.2020.1750607. [CrossRef] [Google Scholar]
- Wan J, Chen B and Rao J. 2020. « Occurrence and Preventive Strategies to Control Mycotoxins in Cereal-Based Food ». Compr Rev Food Sci Food Saf. 19: 928–53. https://doi.org/10.1111/1541-4337.12546. [CrossRef] [Google Scholar]
- Wang Z, Yan MM, Zhang HY, et al. 2014. « 2013 Survey on mycotoxin contamination of feed and raw materials in large-scale farms in Shanghai Pudong area. » Shanghai Veterinary Animal Husbandry Newsletter 59: 53–55. https://doi.org/10.14170/j.cnki.cn31-1278/s.2014.06.022. [Google Scholar]
- Watson S, Gong YY and Routledge M. 2017. Interventions targeting child undernutrition in developing countries may be undermined by dietary exposure to aflatoxin. Crit Rev Food Sci Nutr. 13: 1963–1975. https://doi.org/10.1080/10408398.2015.1040869. [Google Scholar]
- Wen C, Shen M, Liu G, et al. 2023. « Edible vegetable oils from oil crops: Preparation, refining, authenticity identification and application ». Process Biochem. 124: 168–79. https://doi.org/10.1016/j.procbio.2022.11.017. [CrossRef] [Google Scholar]
- Weng MW, Lee HW, Choi B, et al. 2017. « AFB1 hepatocarcinogenesis is via lipid peroxidation that inhibits DNA repair, sensitizes mutation susceptibility and induces aldehyde-DNA adducts at p53 mutational hotspot codon 249 ». Oncotarget. 8: 18213–18226. https://doi.org/10.18632/oncotarget.15313. [Google Scholar]
- Więckowska M, Cichon N, Szelenberger R, et al. 2024. « Ochratoxin A and Its Role in Cancer Development: A Comprehensive Review ». Cancers 16: 3473. https://doi.org/10.3390/cancers16203473. [CrossRef] [PubMed] [Google Scholar]
- Willoquet B, Mirey G, Labat O, et al. 2024. « Roles of cytochromes P450 and ribosome inhibition in the interaction between two preoccupying mycotoxins, aflatoxin B1 and deoxynivalenol ». Sci Total Environ. 955: 176937 https://doi.org/10.1016/j.scitotenv.2024.176937. [Google Scholar]
- Xia Q, Du Z, Lin D, et al. 2021. « Review on contaminants in edible oil and analytical technologies ». Oil Crop Sci. 6: 23–27. https://doi.org/10.1016/j.ocsci.2021.02.001. [CrossRef] [Google Scholar]
- Xu L, Zhang Z, Zhang Q, et al. 2018. « An On-Site Simultaneous Semi-Quantification of Aflatoxin B1, Zearalenone, and T-2 Toxin in Maize- and Cereal-Based Feed via Multicolor Immunochromatographic Assay ». Toxins 10: 87. https://doi.org/10.3390/toxins10020087. [CrossRef] [PubMed] [Google Scholar]
- Zhang C, Qu Z, Hou J, et al. 2024. « Contamination and Control of Mycotoxins in Grain and Oil Crops ». Microorganisms 12: 567. https://doi.org/10.3390/microorganisms12030567. [CrossRef] [PubMed] [Google Scholar]
- Zhang Y, Lin XH, Xia YP, et al. 2018. « Contamination status of some fungal toxins in commercially available vegetable oil in Tianjin in 2017. » Occup and Health 34: 3353–3356. [Google Scholar]
- Zhang Y, Liu C and Van der Fels-Klerx HJ. 2025. « Occurrence, toxicity, dietary exposure, and management of Alternaria mycotoxins in food and feed: A systematic literature review ». Compr Rev Food Sci Food Saf. 24: e70085. https://doi.org/10.1111/1541-4337.700850. [CrossRef] [Google Scholar]
- Zhou J, Yao SS, Wang JM et al. 2023. « Multiple mycotoxins in commonly used edible oils: Occurrence and evaluation of potential health risks ». Food Chem. 426: 136629. https://doi.org/10.1016/j.foodchem.2023.136629. [CrossRef] [Google Scholar]
- Zingales V, Taroncher M, Martino P.A, et al. 2022. « Climate Change and Effects on Molds and Mycotoxins ». Toxins 14: 445. https://doi.org/10.3390/toxins14070445. [CrossRef] [PubMed] [Google Scholar]
- Zio S, Cisse H, Zongo O, et al. 2020. « The Oils Refining Process and Contaminants in Edible Oils: A Review ». J Food Tech Res. 7: 9–47. https://doi.org/10.18488/journal.58.2020.71.9.47. [CrossRef] [Google Scholar]
Cite this article as: Durant A, Pinton P, Puel O, Oswald IP. 2025. Mycotoxins in oilseeds and vegetable edible oils: an overview of toxicity, occurrence and exposure. OCL 32: 19. https://doi.org/10.1051/ocl/2025014.
All Tables
Contribution of edible oils to the chronic dietary exposure to mycotoxins across EFSA risk assessment reports At the LB, results below the limit of quantification (LOQ) or limit of detection (LOD) were replaced by zero; at the upper bound (UB), the results below the LOD were replaced by the value of the LOD and those below the LOQ were replaced by the value reported as LOQ.
All Figures
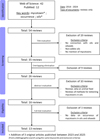 |
Figure 1 Graphical representation of the reviews selection for Tables 1 and 2. |
| In the text | |
Les statistiques affichées correspondent au cumul d'une part des vues des résumés de l'article et d'autre part des vues et téléchargements de l'article plein-texte (PDF, Full-HTML, ePub... selon les formats disponibles) sur la platefome Vision4Press.
Les statistiques sont disponibles avec un délai de 48 à 96 heures et sont mises à jour quotidiennement en semaine.
Le chargement des statistiques peut être long.




