| Numéro |
OCL
Volume 18, Numéro 2, Mars-Avril 2011
Dossier : Vitamines liposolubles
|
|
|---|---|---|
| Page(s) | 76 - 82 | |
| Section | Nutrition – Santé | |
| DOI | https://doi.org/10.1051/ocl.2011.0374 | |
| Publié en ligne | 15 mars 2011 | |
Coenzyme Q 10: multiple benefits in one ingredient
1
Department of Biochemistry, Biology & Genetics, Polytechnic University of the Marche, 60131 Ancona, Italy
2
Kaneka Pharma Europe NV, Brussels, Belgium
Coenzyme Q is a lipid molecule widely diffused in nature; in humans and other mammals it is present as coenzyme Q10. (CoQ10). The first recognized role of CoQ10 was in mitochondrial bioenergetics, where it plays a central role in the production of ATP. It is also present in other subcellular organelles, both in its oxidized and in its reduced state (ubiquinol-10). The reduced form of CoQ10 is endowed with powerful antioxidant activity: it acts as a chain-breaking antioxidant and is also capable of egenerating alpha-tocopherol, the active form of vitamin E. By these mechanisms CoQ10, together with vitamin E, protects lipoproteins from oxidation a process which bears considerable interest in preventing atherosclerosis. CoQ10 has also been found to support cardiovascular function and the latest findings indicate an active role in counteracting endothelial dysfunction, which is closely implicated in cardiovascular disease. CoQ10 also improves sperm motility, an effect which might be related both to its antioxidant and to its bioenergetic properties. Oxidative stress might be involved in neurodegenerative disease, and in migraine, two fields where the positive effects of CoQ10 have been documented. CoQ10 is synthesized by our body but is also present in food and can be taken as a nutritional supplement. The main source of industrially produced CoQ10 is yeast fermentation. The process results in CoQ10 which is identical to the naturally occurring molecule. Ubiquinol, the reduced form of CoQ10, has recently become available.
Key words: Coenzyme Q10 / bioenergetics / antioxidation / skin metabolism / fermentation / nutritional claims
© John Libbey Eurotext 2011
Coenzyme Q (CoQ) also known as ubiquinone, is a lipid molecule widely distributed in nature. In mitochondria, like in other cellular compartments, it is present both in its oxidised state (ubiquinone) and in its reduced one (ubiquinol). The first homolog to be discovered about 50 years ago, in beef mitochondria, was coenzyme Q10 (Crane et al., 1957). In fact, CoQ is made of benzoquinone moiety and an isoprenoid side chain the length of which is 10 units both in man and many mammals; therefore we talk about CoQ10 and reduced CoQ10 (ubiquinol-10). Other living organisms possess different species of CoQ, for instance Saccharomyces cerevisiae produces CoQ6, other microorganisms CoQ7, and many mammals CoQ9. Each organism possesses a dominant homolog of CoQ, and minor amounts of other homologs. Most of CoQ10 available as a food supplement is natural CoQ10, extracted from some microorganisms which synthesize CoQ10, identical to the one which is found in humans and other mammals. This issue will be commented later on in the text.
For a certain number of years CoQ was known for its key role in mitochondrial bioenergetics; later studies demonstrated its presence in other subcellular fractions and in plasma, and extensively investigated its antioxidant role. The rationale supporting the use of CoQ10 as a food supplement is mainly based on these two functions. More recent data reveal that CoQ10 affects the expression of genes involved in human cell signalling, metabolism and transport (Groneberg et al., 2005) and some of the effects of exogenously administered CoQ10 may be due to this property.
New progress has been made in elucidating CoQ10 in metabolism and nutrition. This short chapter is mainly focused on recent findings which will hopefully contribute to better understand the relationship between basic biochemical mechanisms and certain physiological and clinical effects.
CoQ10 and mitochondrial bioenergetics
The essential role of CoQ10 in bioenergetics was postulated since the years of its discovery. In fact several years later, the studies of Nobel Prize winner Peter Mitchell highlighted the central role of this quinone in the chemo-osmotic production of ATP. Therefore CoQ10 is a key component of the mitochondrial machinery, the main energy plant of our cells. At this level it operates as a redox couple (ubiquinone/ubiquinol), responsible for proton and electron transport. If mitochondria are devoid of CoQ10 they cannot produce ATP; in some conditions we can have partial CoQ10 deficiencies.
Even though the concentration of CoQ10 in mitochondria is rather high compared to the corresponding concentration of other mitochondrial components, it is not saturating. This practically means that at the actual concentrations of CoQ10 in these membranes the velocity of the respiratory complexes is not the maximal one. In fact, small variations in the concentration of CoQ10 in these membranes leads to remarkable changes in the respiratory rates of these cells. This can explain why, even though a small part of the exogenously administered CoQ10 is uptaken by our cells, the effect is not negligible (figure 1).
 |
Figure 1. The physiological range of CoQ10 in the mitochondrial respiratory chain is close to the Km, the concentration supporting 50% of the maximum velocity (Source: Estornell E, et al. FEBS Lett 1992; 311 : 107-9). |
Ubiquinone biosynthesis: biochemical and clinical implications
Strictly speaking CoQ10 is not a vitamin, as mammals and lower animals are capable of synthesizing this molecule. A minor part is however introduced through the diet; moreover a series of dietary components is essential for the proper functioning of CoQ10 biosynthesis (figure 2).
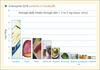 |
Figure 2. Coenzyme Q10 content of different foods (Source: Kamei et al. The Distribution and Content of Ubiquinone in Foods. Internat J Vit Nutr Res 1986; 56: 57-63). |
The synthesis of the quinone moiety of CoQ10 starts from phenylalanine or from tyrosine and the isoprenoid side chain derives from mevalonate. A series of vitamin cofactors is needed for this biosynthesis. According to Karl Folkers the dominant source of CoQ10 in man is biosynthesis. This complex, 17 step process, requiring at least seven vitamins (vitamin B2 – riboflavin, vitamin B3 – niacinamide, vitamin B6, folic acid, vitamin B12, vitamin C, and pantothenic acid) and several trace elements, is, by its nature, highly vulnerable. Karl Folkers argues that suboptimal nutrient intake in man is highly possible and that there is subsequent secondary impairment in CoQ10 biosynthesis. It was highlighted that in a vitamin B6 deficiency plasma CoQ10 levels are also low and they increase upon improvement of the vitamin B6 deficiency status (Willis et al., 1999). In eukaryotes the isoprenoid side chain of coenzyme Q is synthesized through the mevalonate pathway, which also leads to the synthesis of cholesterol. As we will comment below statins, the potent and widely used anticholesterolemic drugs, also inhibit CoQ10 biosynthesis and this could have important practical implications.
Coenzyme Q10 concentration greatly varies in different tissues, probably related to different metabolic demands (figure 3).
 |
Figure 3. Concentration of coenzymeQ10 in different human tissues (Source: Okamoto T, et al. Internat J Vit Nutr Res 59; 288-92; Aberg et al. Archives of Biochemistry and Biophysics and Biophysics 1992; 295: 230-4; Shindo Y, et al. J Invets Dermatol 1994; 102: 122-4. |
Tissue concentrations of CoQ10 also vary with age: for different organs an increase of CoQ10 has been found in the initial decades with a subsequent decrease (figure 4).
 |
Figure 4. The concentration of coenzyme Q10 in the body decreases year by year, indicating that it has a close relationship with aging (Kalen A, et al. Lipids1989; 24: 579). |
CoQ as an antioxidant
In its reduced form (ubiquinol) coenzyme Q acts as a phenolic antioxidant, undergoing hydrogen abstraction by free radicals, therefore it acts like a chain breaking antioxidant. This evidence has been produced by numerous experimental models, both in vivo and in vitro, using artificial membranes, isolated subcellular organelles, cultured cells, isolated perfused organs and clinical models (Dallner and Stocker, 2005).
Ubiquinol may act by slowing down the chain propagation reaction, with a mechanism that is common to the co-called “chain breaking antioxidants”.
Reduced Coenzyme Q is also able to regenerate α-tocopherol, the active form of Vitamin E: in this sense CoQ10 and vitamin E are considered as a lipophilic antioxidant duo of primary importance. In order to act as an antioxidant CoQ must be in the reduced state; several enzymes exert this function of CoQ reductases. There are some conditions where the reducing capacity of the cell might be impaired: in these conditions supplementing CoQ10 already in the reduced state (QH2, ubiquinol-10) might be particularly relevant.
Antioxidant function of CoQ10 in plasma lipoproteins
It is currently believed that high levels of LDL, as well as smoking and hypertension, are primary risk factors, among those contributing to cardiovascular disease. Biochemical mechanisms responsible for the atherogenicity of LDL have been extensively addressed, and experimental evidence bas been produced indicating that oxidatively modified LDL become atherogenic. It was found that endothelial cells are involved in the oxidative attack against LDL. Oxidative attack on LDL deeply affects the apoprotein moiety as well. As a consequence of these changes LDL are no longer “recognized” by the normal receptors, and are taken up more readily by the scavenger receptors of macrophages. LDL leave the blood stream, penetrate the endothelial cell lining and reach the subendothelial space, where they undergo oxidative attack. Oxidatively modified LDL are capable of triggering further events, including platelet activation, and exert a chemotactic attraction on circulating monocytes, which migrate to the subendothelial space, where they become macrophages. These cells have only low levels of the classical LDL receptor, nonetheless they are able to take up more rapidly oxidatively modified LDL, and this uptake involves a different receptor, called the “scavenger receptor”. As discussed above, oxidatively modified LDL are easily recognized by the scavenger receptors. These events lead to an accumulation of lipids, mainly cholesterol and cholesterol esters, in the macrophages, which will become lipid-laden foam cells. Foam cells may be considered the essence of the atheromatous lesions.
LDL are endowed with a number of lipid soluble antioxidants capable of preventing or minimizing lipid peroxidation. Plasma levels of CoQ10 have been extensive investigated (Tomasetti et al., 1999). Most plasma CoQ10 is transported by LDL where, together with vitamin E, it exerts its antioxidant protection. Ubiquinol-10 is the most reactive antioxidant in LDL, and although it is present at lower concentrations compared to vitamin E, it is able to regenerate α-tocopherol from the tocopheril radical, making the vitamin E-ubiquinol duo the most important antioxidant system in LDL. CoQ10 enriched LDL, isolated from plasma of healthy volunteers orally treated with CoQ10 for a few days, were less susceptible to peroxidizability in vitro, compared to the same LDL in basal conditions (Mohr et al., 1992).
Blood CoQ10 is mainly transported by LDL, although it is also present in the other classes of lipoproteins and in blood cells. Its concentration is usually reported in micrograms/litre of plasma or micromoles/litre. But it is worthwhile to normalize these values according to the blood LDL content or at least to plasma cholesterol levels. The CoQ10/total cholesterol level could have a predictive value in cardiovascular disease (Molyneux et al., 2008). Besides decreasing LDL peroxidizability, CoQ10 could have a direct antiatherosclerotic effect, in fact animal studies have shown that CoQ10 administration attenuates aortic atherosclerotic lesions (Witting et al. 2000; Singh et al. 2000).
CoQ10: analytical aspects
CoQ10 is commonly assayed in plasma, both in basal conditions and after oral supplementation. Basal CoQ10 levels might reflect CoQ10 deficiency and, as pointed out above, they might have a predictive value in cardiac failure. Post supplementation levels of CoQ10 are also important, since a clinical response is much more common if some threshold values are reached. Several studies have highlighted that a plasma level of at least 2.5 μg/mL should be reached to have a consistent physiological response (Belardinelli et al., 2006). Of course quantification of plasma CoQ10 is also important to assess bioavailability of different CoQ10 formulations. Methods are usually based on HPLC separation: a simple, yet precise and accurate method is the one which appears in the website of the International CoQ10 Association (Littarru et al., 2004).
Coenzyme Q10 can also be quantitatively assayed in cells and in biological fluids. CoQ10 cellular levels are particularly important in some “primary CoQ10 deficiencies”. These are conditions where, due to genetic reasons one or more of the steps involved in CoQ10 biosynthesis are impaired. In some cases there is a dramatic positive response to exogenous CoQ10 administration (Quinzii et al., 2008).
Some analytical problems have been found in the quantification of CoQ10 (and other CoQs) in vegetable oils and generally in fatty samples, due to interferences mainly with triacylglycerides. A clean, efficient separation and quantification procedure was recently proposed and applied to the determination of CoQ9 and CoQ10 contents in different vegetable oil samples (Rodriguez-Acuna et al., 2008).
Physiological effects: CoQ10 and physical exercise
The key role of coenzyme Q10 in mitochondrial bioenergetics has suggested its use in an attempt to improve aerobic capacity and physical performance. Some studies have highlighted an ergogenic effect while others did not. These issues have recently been addressed in 3 papers published in 2008 (Cooke et al., 2008, Mizuno et al., 2008, Kon et al., 2008). One of these articles shows that following a single administration of CoQ10 plasma levels significantly correlated with muscle CoQ10 levels, maximal oxygen consumption and treadmill time to exhaustion. A trend for increased time to exhaustion was observed following two weeks of CoQ10 supplementation (p = 0.06) (Cooke et al., 2008). In another trial, oral administration of CoQ10 improved subjective fatigue sensation and physical performance (Mizuno et al., 2008). The third article is a double blind study where a group of kendo athletes showed lower levels of CK, myoglobin and lipid peroxides compared to the corresponding values in the placebo group (Kon et al., 2008).
In a study where CoQ10 had been taken in combination with vitamin C and E, administration of this antioxidant cocktail further increased the eNOS and uncoupling protein 3 (UCP3) mRNA content after exercise (Hellsten et al., 2007).
For the first time a study examined the acute effects of CoQ10 and placebo on autonomic nervous activity and energy metabolism at rest and during exercise (Zheng and Moritani, 2008). Fat oxidation significantly increased during exercise in the CoQ10 group; results suggested that CoQ10 increases autonomic nervous activity during low intensity exercise.
In a double blind pilot study patients with post-polio syndrome were treated with 200 mg of CoQ10/day. Muscle strength, muscle endurance and quality of life increased statistically significantly in all 14 patients but there was no significant difference between the CoQ10 and placebo groups (Kough et al., 2008).
Effects on skin metabolism
The bioenergetic and antioxidant properties of CoQ10 have also been studied at skin level. The first report was by Hoppe et al. (1999). This paper demonstrated that CoQ10 penetrates into the viable layers of the epidermis and reduces the levels of oxidation measured by weak photon emission. CoQ10 was also effective in human keratinocytes against UVA mediated oxidative stress and in suppressing the expression of collagenase in human dermal fibroblasts following UVA irradiation. A reduction in wrinkle depth following CoQ10 application was also shown, an effect confirmed by Ashida et al. (2005). The combined effect of creatine and CoQ10 on skin’s energy metabolism was highlighted by Blatt et al. (2005). Recently Inui and collaborators showed that cytokine production in keratinocytes is inhibited by CoQ10, resulting in a decrease of metalloproteinases leading to wrinkle reduction.
Reproductive medicine
Impairment of mitochondrial bioenergetics and oxidative stress are known to be involved in sperm motility. After a series of studies highlighting the implications of CoQ10 in male infertility a more recent publication confirmed, in a placebo controlled double-blind randomized trial, the efficacy of CoQ10 treatment in improving semen quality in men with idiopathic infertility (Balercia et al., 2009). Oxidized and reduced CoQ10 concentration significantly increased both in seminal plasma and sperm cells, together with sperm motility, after 6 months of therapy with 200 mg/day CoQ10. The increased concentration of CoQ10 and QH2 (reduced CoQ10) in seminal plasma and sperm cells, the improvement of semen kinetic features and treatment, and the evidence of a direct correlation between CoQ10 concentrations and sperm motility strongly support a cause-effect relationship. Similar results were found by Safarinejad (2009). In this study 212 infertile men with idiopathic oligoasthenoteratospermia were treated with 300 mg/day CoQ10 or placebo for 26 weeks. Statistically significant improvement was found, in the CoQ10 group, regarding sperm count and motility values, with a positive correlation between treatment duration of CoQ10 and sperm count as well as mean sperm motility. The CoQ10 group had a significant decrease in serum FSH and LH at the 26 week treatment phase. The authors highlight that a lower serum FSH implies a better spermatogenesis. Moreover, Inhibin B, which reflects Sertoli’s cell function, increased in the CoQ10 group.
These studies did not address the key issue of pregnancy rate; they were simply aimed at determining an effect of CoQ10 on sperm motility and quality. Other variables should of course be taken into account in order to determine whether CoQ10 has an influence on pregnancy rate.
CoQ10 supports cardiovascular function
CoQ10 deficiency at myocardial level has been documented in different studies. Although in most cases the deficiency was not the cause of the cardiopathy this might have contributed to the severity of the disorder. Numerous trials have been conducted on the effect of CoQ10 as coadjuvant in the treatment of cardiac failure. In many cases quality of life, clinical symptoms and the frequency of hospitalization were ameliorated upon CoQ10 administration. In some protocols there was also an improvement of ejection fraction and other functional parameters.
Cardiovascular effects of CoQ10 can be ascribed to its bioenergetic role, to its capability of antagonizing oxidation of plasma LDL and to its effect in ameliorating endothelial function. This effect was first seen by Watts et al. in patients affected by Type II diabetes (Watts et al., 2002) and then further explored by Belardinelli et al. in patients affected by ischemic heart disease (Belardinelli et al., 2006). Endothelial dysfunction is commonly believed as an early sign of vascular impairment and the capability of CoQ10 in counteracting it represents a promising field. The mild hypotensive effect of CoQ10 is probably related to this property.
Human CoQ10 deficiencies
Already in the past CoQ10 had been shown to be effective in a number of cases of mitochondrial myopathies, which were sometimes associated with low CoQ10 muscle levels. With the progress in molecular biology techniques primary CoQ10 deficiencies, due to mutations in ubiquinone biosynthetic genes, have been identified and some of these syndromes have shown excellent responses to oral CoQ10 treatment (Quinzii et al., 2008).
Statins and CoQ10
Statins are 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors which decrease synthesis of mevalonate, a key metabolic step in the cholesterol synthesis pathway. These efficient drugs can produce a variety of muscle-related complaints or myopathies. Since the mevalonate pathway also leads to the biosynthesis of the isoprenoid side chain of coenzyme Q10, different studies have addressed the possibility of CoQ10 being an etiologic factor in statin myopathy. There is no doubt that statins decrease plasma and leukocyte CoQ10 levels; a few studies also report a decrease of muscle CoQ10 level upon statin treatment. This controversial issue has been extensively investigated (Littarru and Langsjoen, 2007; Marcoff and Thompson, 2007). A small-sized, yet double-blind study also points out that CoQ10 exogenous administration reduced myopathic symptoms in statin treated patients (Caso et al., 2007). Of course a large double blind clinical trail would be necessary in order to assess the capability of CoQ10 in mitigating statin side effects.
Neurodegenerative disease
The positive effect of oral administration of CoQ10 to patients affected by Parkinson’s disease was investigated in 2002 by Shults et al. (2002). Friedreich’s ataxia is another condition where treatment with CoQ10 and vitamin E caused a prolonged improvement in cardiac and skeletal muscle bioenergetics and clinical scores (Cooper and Schapira, 2007).
In 2005 Sandor et al. studied the effect of CoQ10 (300 mg/day for 3 months) in 42 migraine patients in a double-blind, randomized, placebo-controlled trial. The primary outcome variable in this study was a change of attack frequency in the third month of treatment compared to baseline. The authors showed that responders were 47.6% in the CoQ10 treated group vs 14.4% in the placebo group. A positive effect of CoQ10 was also demonstrated in a large group of pediatric patients suffering from migraine (Hershey et al., 2007).
Sourcing: main natural origins, Kaneka’s process (yeast fermentation)
There are 3 methods used for the manufacturing of CoQ10: yeast fermentation, bacteria fermentation and chemical synthesis. The latter was the first industrial method, introduced by Nisshin in the early 70s. Greater amounts of CoQ10 became available when Japan-based Kaneka Corporation began producing natural CoQ10 (also called KANEKA Q10™) via patented yeast fermentation in 1977. Kaneka is now the world’s largest manufacturer of CoQ10 and is the only producer to manufacture CoQ10 in US market.
The yeast-fermentation method, along with Kaneka’s rigorous manufacturing standards, makes KanekaQ10™ the purest commercial-grade CoQ10 available on the market today. The process results in CoQ10 with the so-called all-trans configuration, which means that it is identical to naturally occurring CoQ10 found in meat, fish and other products and also bio-identical to CoQ10 produced in the human body (figure 5).
 |
Figure 5. The trans and cis isomers of coenzyme Q10. |
The Kaneka yeast fermentation process is in accordance with pharmaceutical GMP standards and does not contain impurities found in synthetic material.
Kaneka Q10TM is the only CoQ10 backed by published human safety studies and is the primary CoQ10 used in most scientific studies. As purest, most rigorously tested CoQ10 available, KanekaQ10 has been used in all major CoQ10 clinical trials approved by the FDA and funded by the NIH (e.g. Phase III Clinical Trial on Coenzyme Q10’s Effects on Huntington’s and Parkinson Disease in US).
Regulatory status
CoQ10 is a well-established ingredient that is present in many food supplements, fortified foods and cosmetic brands all over Europe. There is an increasing acceptance of the role of non-vitamin and mineral ingredients, such as CoQ10 and its levels move towards higher levels than were considered a decade ago based on risk assessment approach and supportive data. A Belgian ministerial order determined CoQ10 was safe for use in food supplements at 200 mg after reviewing a dossier of Kaneka that provided scientific arguments to substantiate the safety of CoQ10 up to 200 mg/day and higher. The 200 mg level is being followed by other European Union countries, and potentially all of them, by the legal principle of new Mutual Recognition Regulation. This Regulation requires products lawfully marketed in one EU member state to be permitted entry into another member state’s market. The mutual recognition regulation plays a key role in the future EU market for CoQ10.
According the new European Health Claim regulation (EC 1924/2006) the generic health claims were planned to be disclosed in a positive list that EU would give access to by end of January 2010. Yet, early 2011 there is still no list available. In the meanwhile, national regulation still applies and all health claims that are well supported by science can continue to be used.
EFSA has started to deliver scientific opinions on art. 13 regarding generic health claims. EFSA is taking the stance to treat Vitamins and Minerals very differently from Other Substances - examples of the latter are CoQ10, glucosamine, lutein, lycopene, carnitine, etc. For Vitamins and Minerals a “scientific consensus” is usually sufficient to substantiate a health claim, whereas for Other Substances, golden standard human trials are required with conclusive evidence of cause and effect. Moreover, for Vitamins and Minerals EFSA accepts “textbook” knowledge as evidence.
As a general rule, EFSA does not accept human studies conducted on patients, yet medicinal paradigms are expected. Unfortunately, up to now, many CoQ10 health benefits (as for other nutraceuticals) have been studied in patients. Because of this evolution, most generic claims for Other Substances have not been accepted by EFSA. This is also true for the generic CoQ10 claims: energy, antioxidant, and blood pressure normalizing. We are facing a situation where study designs, which are acceptable in the scientific community, are not usable for marketing purposes.
Only in 2011 and 2012, which is more than 4 years after publication of the health claim regulation, EFSA will organise guidance meetings and publish documents to provide more clarity on their idea of how health claims should be substantiated for different fields such as weight management, cardiovascular health, joint health, physical performance, etc. Guidelines specifying the type of studies, the proper biomarkers to be used and other pertinent issues should also be established. The scientific community is just beginning to come to terms with health claim regulations and this process is ongoing. The full impact of the regulations is still evolving and many grey areas are apparent. An independent economic impact assessment of existing and potential effects of the health claims regulation concluded that all initial objectives of the health claims regulation, notably relating to objectives such as consumer protection, fair competition, and promotion of R&D, were only poorly or weakly addressed (Brookes, 2010).
Ubiquinol
Ubiquinol, the reduced form of CoQ10 has recently become available in stable form, and is manufactured exclusively by Kaneka. Ubiquinol represents 93-95% of CoQ10 pool in plasma of healthy human and is the predominant Coenzyme Q10 form in a healthy cell. Several studies suggest that ubiquinol-ratio in human plasma may represent a sensitive index of oxidative stress in vivo especially indicative of early oxidative damage. CoQ10 researchers from around the world are working to advance the understanding of the newly available ubiquinol. Recently, Japanese researchers showed protective effects of ubiquinol on influenza virus infection in mice.
Conclusion
CoQ10 is a highly studied nutrient whose biochemical and physiological role has been established. What is special about this molecule is its involvement both in the bioenergetic and in the antioxidant processes. While waiting for a definite pronunciation by the European authorities, national regulations still apply and the number of health claims that are well supported by science can continue to be used.
References
- Ashida Y, Yamanishi H, Terada T, Oota N, Sekine K, Watabe K. CoQ10 supplementation elevates the epidermal CoQ10 level in adult hairless mice. Biofactors 2005; 25: 1758. [CrossRef] [Google Scholar]
- Balercia G, Buldreghini E, Vignini A, et al. Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: a placebo-controlled, double-blind randomized trial. Fertil Steril 2009; 91: 1785–1792. [CrossRef] [PubMed] [Google Scholar]
- Belardinelli R, Mucaj A, Lacalaprice F, et al. Coenzyme Q10 and exercise training in chronic heart failure. Eur Heart J 2006; 27: 2675–2681. [CrossRef] [PubMed] [Google Scholar]
- Blatt T, Lenz H, Koop U, et al. Stimulation of skin’s energy metabolism provides multiple benefits for mature human skin. Biofactors 2005; 25: 179–185. [CrossRef] [PubMed] [Google Scholar]
- Brookes G. Economic Impact Assessment of the European Union (EU)’s Nutrition & Health Claims Regulation on the EU food supplement sector and market for the European Health Claims Alliance (EHCA). 2010. [Google Scholar]
- Caso G, Kelly P, Mcnurlan MA, Lawson WE. Effect of coenzyme Q10 on myopathic symptoms in patients treated with statins. Am J Cardiol 2007; 99: 1409–1412. [CrossRef] [PubMed] [Google Scholar]
- Cooke M, Iosia M, Buford T, et al. Effects of acute and 14-day coenzyme Q10 supplementation on exercise performance in both trained and untrained individuals. J Int Soc Sports Nutr 2008; 4 (5): 8. [CrossRef] [Google Scholar]
- Cooper JM, Schapira AH. Friedreich’s ataxia: coenzyme Q10 and vitamin E therapy. Mitochondrion 2007; 7: S127–S135. [CrossRef] [PubMed] [Google Scholar]
- Crane FL, Hatefi Y, Lester RL, Widmer C. Isolation of a quinone from beef heart mitochondria. Biochim Biophys Acta 1957; 25: 220–221. [CrossRef] [PubMed] [Google Scholar]
- Dallner G, Stocker R, Coenzyme Q. In: Encyclopedia of dietary supplements. New York: Marcel Dekker, 2005: 121–131. [Google Scholar]
- Groneberg DA, Kindermann B, Althammer M, et al. Coenzyme Q10 affects expression of genes involved in cell signalling, metabolism and transport in human CaCo-2 cells. Int J Biochem Cell Biol 2005; 37: 1208–1218. [CrossRef] [PubMed] [Google Scholar]
- Hellsten Y, Nielsen JJ, Lykkesfeldt J, et al. Antioxidant supplementation enhances the exercise-induced increase in mitochondrial uncoupling protein 3 and endothelial nitric oxide synthase mRNA content in human skeletal muscle. Free Radic Biol Med 2007; 43: 353–361. [CrossRef] [PubMed] [Google Scholar]
- Hershey AD, Powers SW, Vockell AL, et al. Coenzyme Q10 deficiency and response to supplementation in pediatric and adolescent migraine. Headache 2007; 47: 73–80. [CrossRef] [PubMed] [Google Scholar]
- Hoppe U, Bergemann J, Diembeck W, et al. Coenzyme Q10, a cutaneous antioxidant and energizer. Biofactors 1999; 9: 371–378. [CrossRef] [PubMed] [Google Scholar]
- Kon M, Tanabe K, Akimoto T, et al. Reducing exercise-induced muscular injury in kendo athletes with supplementation of coenzyme Q10. Br J Nutr 2008; 100: 903–909. [PubMed] [Google Scholar]
- Kough K, Krossen C, Heiwe S, Theorell H, Borg K. Effects of resistance training in combination with coenzyme Q10 supplementation in patients with post-polio: a pilot study. J Rehabil Med 2008; 40: 773–775. [PubMed] [Google Scholar]
- Littarru GP, Mosca F, Fattorini D, Bompadre S, Battino M. Assay of coenzyme Q10 in plasma by a single dilution step. Methods Enzymol 2004; 378: 170–176. [CrossRef] [PubMed] [Google Scholar]
- Littarru GP, Langsjoen P. Coenzyme Q10 and statins: biochemical and clinical implications. Mitochondrion 2007; 7: S168–S174. [CrossRef] [PubMed] [Google Scholar]
- Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol 2007; 49: 2231–2237. [CrossRef] [PubMed] [Google Scholar]
- Mizuno K, Tanaka M, Nozaki S, et al. Antifatigue effects of coenzyme Q10 during physical fatigue. Nutrition 2008; 24: 293–299. [CrossRef] [PubMed] [Google Scholar]
- Mohr D, Bowry VW, Stocker R. Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochim Bio- phys Acta 1992; 1126: 247–254. [CrossRef] [Google Scholar]
- Molyneux SL, Florkowski CM, George PM, et al. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. J Am Coll Cardiol 2008; 52: 1435–1441. [CrossRef] [PubMed] [Google Scholar]
- Quinzii CM, Lopez LC, Naini A, Dimauro S, Hirano M. Human CoQ10 deficiencies. Biofactors 2008; 32: 113–118. [CrossRef] [PubMed] [Google Scholar]
- Rodriguez-Acuna R, Brenne E, Lacoste F. Determination of coenzyme Q10 and Q9 in vegetable oils. J Agric Food Chem 2008; 56: 6241–6245. [PubMed] [Google Scholar]
- Safarinejad MR. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J Urol 2009; 182: 237–248. [CrossRef] [PubMed] [Google Scholar]
- Shults CW, Oakes D, Kieburtz K, et al. (Parkinson Study Group). Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol 2002; 59: 1541–1550. [CrossRef] [PubMed] [Google Scholar]
- Singh RB, Shinde SN, Chopra RK, Niaz MA, Thakur AS, Onouchi Z. Effect of coenzyme Q10 on experimental atherosclerosis and chemical composition and quality of atheroma in rabbits. Atherosclerosis 2000; 148: 275–282. [CrossRef] [PubMed] [Google Scholar]
- Tomasetti M, Alleva R, Solenghi MD, Littarru GP. Distribution of antioxidants among blood components and lipoproteins: significance of lipids/CoQ10 ratio as a possible marker of increased risk for atherosclerosis. Biofactors 1999; 9: 231–240. [CrossRef] [PubMed] [Google Scholar]
- Watts Gf, Playford Da, Croft Kd, Ward Nc, Mori Ta, Burke V. Coenzyme Q(10) improves endothelial dysfunction of the brachial artery in Type II diabetes mellitus. Diabetologia 2002; 45: 420–426. [CrossRef] [PubMed] [Google Scholar]
- Willis R, Anthony M, Sun L, Honse Y, Qiao G. Clinical implications of the correlation between coenzyme Q10 and vitamin B6 status. Biofactors 1999; 9: 359–363. [CrossRef] [PubMed] [Google Scholar]
- Witting PK, Pettersson K, Letters J, Stocker R. Anti-atherogenic effect of coenzyme Q10 in apolipoptrotetin E gene knockout mice. Free Radic Biol Med 2000; 29: 295–30. [CrossRef] [PubMed] [Google Scholar]
- Zheng A, Moritani T. Influence of CoQ10 on autonomic nervous activity and energy metabolism during exercise in healthy subjects. J Nutr Sci Vitaminol 2008; 54: 286–290. [CrossRef] [Google Scholar]
To cite this article: Littarru GP, Lambrechts P. Coenzyme Q10: multiple benefits in one ingredient. OCL 2011; 18(2): 76–82. doi: 10.1051/ocl.2011.0374
All Figures
 |
Figure 1. The physiological range of CoQ10 in the mitochondrial respiratory chain is close to the Km, the concentration supporting 50% of the maximum velocity (Source: Estornell E, et al. FEBS Lett 1992; 311 : 107-9). |
| In the text | |
 |
Figure 2. Coenzyme Q10 content of different foods (Source: Kamei et al. The Distribution and Content of Ubiquinone in Foods. Internat J Vit Nutr Res 1986; 56: 57-63). |
| In the text | |
 |
Figure 3. Concentration of coenzymeQ10 in different human tissues (Source: Okamoto T, et al. Internat J Vit Nutr Res 59; 288-92; Aberg et al. Archives of Biochemistry and Biophysics and Biophysics 1992; 295: 230-4; Shindo Y, et al. J Invets Dermatol 1994; 102: 122-4. |
| In the text | |
 |
Figure 4. The concentration of coenzyme Q10 in the body decreases year by year, indicating that it has a close relationship with aging (Kalen A, et al. Lipids1989; 24: 579). |
| In the text | |
 |
Figure 5. The trans and cis isomers of coenzyme Q10. |
| In the text | |
Les statistiques affichées correspondent au cumul d'une part des vues des résumés de l'article et d'autre part des vues et téléchargements de l'article plein-texte (PDF, Full-HTML, ePub... selon les formats disponibles) sur la platefome Vision4Press.
Les statistiques sont disponibles avec un délai de 48 à 96 heures et sont mises à jour quotidiennement en semaine.
Le chargement des statistiques peut être long.




