| Numéro |
OCL
Volume 32, 2025
Minor oils from atypical plant sources / Huiles mineures de sources végétales atypiques
|
|
|---|---|---|
| Numéro d'article | 23 | |
| Nombre de pages | 13 | |
| DOI | https://doi.org/10.1051/ocl/2025018 | |
| Publié en ligne | 16 juillet 2025 | |
Research Article
Bioactive compounds and oxidative stability of the oils from white, red, and black rice brans obtained by modified three-phase partitioning☆
1
Food Science Doctoral Program, Faculty of Agricultural Technology, Universitas Brawijaya, Jalan Veteran Malang 65145, Indonesia
2
Department of Food Science and Biotechnology, Faculty of Agricultural Technology, Universitas Brawijaya, Jalan Veteran Malang 65145, Indonesia
3
Study Center for Local Food Development, Universitas Brawijaya, Jawa Timur 65145, Indonesia
* Corresponding author: teties@yahoo.co.id;teties@ub.ac.id
Received:
16
January
2025
Accepted:
3
June
2025
Rice bran oil (RBO) is valued for its bioactive compounds, which are beneficials for food and health. The oil characteristics from pigmented rice brans, such as red and black, are still limitedly explored. This study investigates RBO extracted from white (WRB), red (RRB), and black (BRB) rice brans using modified three-phase partitioning (TPP). The highest rice bran oil yield is observed at 2 hours of extraction for all rice brans, demonstrating the best oxidative stability. All rice bran oils had linoleic acid as the dominant fatty acid. The advantage of rice bran oil is the presence of bioactive compounds, including phenolics, flavonoids, phytosterols, tocotrienols, and γ-oryzanol. Black rice bran oil had the highest phenolics, anthocyanins, γ-oryzanol, and tocotrienols, contributing to its highest oxidative stability. The highest phytosterols level was found in red rice bran oil. Meanwhile, white rice bran oil had the superiority due to the highest flavonoid content. This study provides some insights about characteristics of pigmented rice bran oils for future applications.
Key words: γ-oryzanol / oxidative stability / phytosterols / rice bran oil / tocotrienols
© Miftahurrahmi et al., Published by EDP Sciences, 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Highlights
Modified three-phase partitioning is suitable for extracting rice bran oil as a part of consecutive rice bran macro components separation.
Rice bran with different colors reveals different oil characteristics.
Black rice bran oil exhibits the highest oxidative stability, contributed by phenolics, anthocyanins, γ-oryzanol, and tocotrienols.
1 Introduction
Rice bran is the by-product of rice milling and polishing, which consists of pericarp, aleurone, and sub-aleurone (Sapwarobol et al., 2021). The brown outer layer of the rice grain is rice bran, consisting of carbohydrates, lipids, fiber, protein, oryzanols, unsaturated fatty acids, and phenolic compounds. The high nutritional value and bioactive compounds of rice bran are currently gaining attention for obtaining health benefits such as treatment against cancer, diabetes, and inflammation (Manzoor et al., 2023). The abundance of rice bran is a challenge to explore for its added value. The US Department of Agriculture (USDA) projected that global rice production reached 503.6 million tons in 2022–2023 (Nidhishree et al., 2024), at least 78.9 million tons of rice bran are being produced (Morales-Ramos et al., 2020).
Rice bran oil (RBO) is a constituent of rice bran produced commercially and available in the market for several purposes, such as cooking oil. This oil is unique due to its unique fatty acid composition, and contains γ-oryzanol and vitamin E (Punia et al., 2021). About 15-22% of oil can be found in rice bran (Balachandran et al., 2008). The biggest RBO production is from India and China, followed by Japan and Thailand. RBO total production can reach more than 1.2 million tons, and the trend is expected to increase over the years ahead. In general, RBO contains around 22% saturated fatty acids, 43% monounsaturated fatty acids, and 35% polyunsaturated fatty acids (Lai et al., 2019).
Saturated fatty acids are found to be high in RBO, around 18.4–25.5% (Lai et al., 2019). As an edible oil, a higher percentage of saturated fatty acids can be a drawback as it affects blood cholesterol levels. On the contrary, RBO has a lowering cholesterol function. This property is affected by unsaponifiable compounds, such as gamma-oryzanol, tocopherol, and tocotrienol, compared to its fatty acid composition (Pal and Pratap, 2017; Ali et al., 2023).
The components of rice bran are candidates for valuable food ingredients. Separation of oil, protein, starch, and fiber, both soluble and insoluble, from rice bran by a simultaneous preparation will produce potential ingredients efficiently. One method has been explored to obtain all rice bran components is three-phase partitioning (TPP) (Wang et al., 2020). TPP is a method for the separation and purification of biomolecules, where the partitioning into three phases depends mainly on the concentration of alcohol and salt used (Chew et al., 2018). The study of Wang et al. (2020) revealed the optimal conditions to obtain rice bran oil (RBO), rice bran protein (RBP), and rice bran polysaccharides (RBPS) was ammonium sulphate concentration of 28%, slurry to t-butanol ratio of 1:1.1 (v/v), extraction temperature of 40 °C, pH 5.10, and extraction time 1 h, which yielded RBP, RBO, and RBPS of 6.81%, 17.28%, and 2.09%, respectively.
Pigmented rice is gaining popularity due to its higher bioactive compounds, health benefits, and biological activities (Bhat et al., 2020), leading to increased production of pigmented rice bran. RBO from pigmented rice bran is supposed to possess superiority due to more bioactive compounds. Modified TPP enables simultaneous extraction and separation of rice bran components, including RBO, RBP, rice bran starch, rice bran fiber, and the pigment from pigmented rice brans in one process. This method uses multiple organic solvents in one step of extraction to obtain a three-phase extract that consists of the upper phase (organic solvents and oil), middle phase (water, protein, soluble dietary fiber, pigments), and lower phase (starch and insoluble dietary fiber).
This study examines a modified TPP to isolate rice bran oil (RBO) from red and black rice, in comparison to white rice bran. Typically, the TPP method utilizes t-butanol combined with ammonium sulfate ((NH₄)₂SO₄), resulting in a top layer rich in oil and a precipitate containing proteins (Chew et al., 2018; Liu et al., 2019; Wang et al., 2020). However, n-hexane, being significantly more nonpolar than t-butanol, dissolves neutral triglycerides more efficiently, where conventional hexane extraction generally recovers over 95% oil (Peng et al., 2021). In contrast, the partial polarity of t-butanol limits lipid solvation. Furthermore, (NH₄)₂SO₄ creates a strong “salting-out” effect on proteins, starch, and fiber, which aids in protein purification but is unnecessary when the goal is solely oil extraction. By omitting the salt and using only water and n-hexane, the process is streamlined into two phases: hexane rapidly captures nearly all the oil in the organic layer, while proteins and insoluble solids remain in the aqueous phase. This modification simplifies the recovery process and improves oil yield, which is the primary goal when extracting rice bran oil, compared to traditional TPP methods that focus on native protein partitioning.
The RBO from pigmented rice bran is still limitedly studied for its physicochemical characteristics, methods of extraction, and the occurrence of bioactive compounds. This study aimed to apply modified TPP to separate oil from white, red, and black rice bran and characterize the extracted oil, including physicochemical properties, oxidative stability, and bioactive compounds. Modified TPP was performed in this study as part of a series of major constituents of rice bran separation to firstly separate oil in the upper layer, protein, pigments, and soluble dietary fiber in the middle layer, and starch and insoluble dietary fiber in the bottom layer. Therefore, modification with the use of hexane for oil extraction and not using ammonium sulfate to precipitate protein aimed to obtain all components of the rice bran in one series of separations. The difference with traditional solvent extraction was that in the modified TPP, the RBO was mixed with water and solvent in the ratio of 1:1 (v/v). The drawbacks of solvent extraction are that it is performed in high temperatures, and the residual can not be used for other rice bran macro component separation
2 Materials and methods
2.1 Materials
Commercial non-glutinous white, red, and black rice bran was obtained from rice milling at Ngawi (white and black rice bran) and Salatiga (black rice bran) Regency, Indonesia, which was sold offline and online. These milling units function as rentals, processing various paddy types provided by farmers. Therefore, the specific rice cultivars examined in this study are not defined and reflect typical regional landraces or mixed cultivars frequently cultivated in these region, and the bran is sold commercially. Therefore we used the term of commercial rice bran to represent this conditions. Rice brans were sieved through a 100-mesh sieve, sealed, and stored −20 °C before analysis. No stabilization steps were done for samples preparation. Chemicals used for analysis are hexane (technical grade), methanol, iron (II), ammonium thiocyanate, n-hexane, acetic acid glacial, NaOH, phenolphthalein, Folin-Ciocalteu, sodium carbonate, BF3-methanol, etc. The abovementioned reagents were analytical grade, unless stated otherwise.
2.2 Modified TPP to separate RBO
Modified TPP was done by mixing 20 g of rice bran with 100 ml distilled water and 100 ml hexane for 1, 1.5, and 2 hours at 40 °C and 600 rpm, then centrifuged at 6000 rpm for 10 min (Wang et al., 2020 with modifications). The extraction was performed only once. The upper phase was pooled, and the hexane was evaporated using a rotary evaporator for 5 min at 40 °C. The yields were recorded, and the RBO was transferred into a dark glass bottle and stored at around −20 °C. The treatments were performed in three replications.
The extracted oil from each 20 g sample of rice bran, which typically yielded between 1 and 2.8 g of oil (representing a 5–14% yield), was promptly weighed and placed into 2 ml amber glass vials with caps to reduce light and oxygen exposure. Multiple replicates were performed for each extraction to ensure enough pooled oil for all analyses. After pooling, the oil was gently shaken to homogenize and then divided into separate vials assigned for different time points in the oxidative stability test. The peroxide value, p-anisidine value, and total oxidation (TOTOX) value were determined at intervals of 0, 7, 14, 21, and 28 days. The highest yield and oxidative stability determined the best extraction time. The oil obtained from the best extraction time was then analyzed for its color, free fatty acid content, acid value, total phenolics, flavonoids, anthocyanins content, fatty acid profile, gamma-oryzanol, phytosterols, and vitamin E. The extraction at the best time was conducted with three repetitions.
2.3 Oxidative stability analysis
The oil was stored in a dark bottle with tightly capped and placed in an incubator at 60 °C, and the oxidation rate was measured by peroxide (Mehta et al., 2015), p-anisidine (Deepika et al., 2014 with modifications), and total oxidation value (Matthäus, 2010) for 28 days. The accelerated oxidative stability analysis was conducted at a temperature of 60 °C, and sampling was performed every 7 days, starting from day 0. Heating for 28 days was sufficient to describe the oxidative stability of RBO. The extraction produced the highest yield for each rice bran, which was determined as the best extraction time, and the oil was subjected to physicochemical and oxidative stability analysis.
2.4 Color analysis
Color analysis (Khan et al., 2009) of RBO from the best extraction time, using a color reader Konica Minolta CR-10 (Tokyo, Japan), which includes L, a, b, C, and h.
2.5 Free fatty acid (FFA) and acid value (AV) analysis
FFA and acid AV were analyzed in RBO from the best extraction time, using the method from Franklin et al. (2020).
2.6 Total phenolics, flavonoids, and anthocyanins content
The analysis was conducted for the RBO of the white, red, and black rice bran from the best extraction time. TPC was determined using the Folin-Ciocalteu method (Sharma et al., 2011), TFC was determined using a modified method (Marinova et al., 2005), and TAC was determined using the pH differential method (Wrolstad and Giusti, 2001).
2.7 Fatty acid profile analysis
Fatty acid profile analysis was conducted for the RBO of the white, red, and black rice bran from the best extraction time. RBO was esterified by the in situ transesterification method (Park and Goins, 1994) to FAME (fatty acid methyl ester). The fatty acid composition was analyzed using a GC Agilent Technologies 7890B (USA) equipped with a flame ionization detector (FID). The amount of 1 μl of samples was injected into the GC apparatus. The analytical column was an HP-88 (USA) (100 m × 0.3 mm × 0.2 μm film thickness) from Agilent, Inc. The column oven temperature started at 100 °C with a 5 min hold time, then 240 °C at 4 °C/min and hold for 15 min, with the total running time of 55 min. The analysis was done by split injection at a 10 to 1 ratio. Helium (He) and nitrogen (N2) were the carrier and makeup gas, respectively. The temperature of the injector and detector was kept at 260 °C. Comparisons between standards and sample retention times were made to determine the samples’ FAME. FAME standards containing 37 fatty acid mixtures were obtained from Supelco, Inc.
2.8 Analysis of gamma-oryzanol, phytosterols, and tocols
Bioactive compounds were analyzed for the RBO of the white, red, and black rice bran from the best extraction time.
2.8.1 Sample preparation
Sample preparation of RBO was performed following the method of Mo et al. (2013) method with slight modifications. Briefly, 60 mg RBO was mixed with 2 ml ethanolic KOH 2 M and heated at 80 °C for 60 mins. Then, 2 ml of deionized water and 3 ml of n-hexane were mixed thoroughly and centrifuged to obtain the upper layer. The extraction process was repeated twice more. The evaporation was then carried out with nitrogen gas, and the residue was reconstituted in 2 mL of ethanol. The samples were then filtered with a 0.45 μm filter paper and stored in −20 °C, waiting for HPLC analysis.
2.8.2 Analysis of gamma-oryzanol
The gamma-oryzanol was measured using HPLC Shimadzu LC 20AT (Japan) with SPD-40/40V detector (Japan) and Shim-pack VP ODS 5 μm 150 × 4.6 mm column (Japan). The column temperature was set to 40 °C using isocratic mobile phase (methanol:acetonitrile:isopropanol, 50%:40%:10%) with 1 ml/min flow rate and 20 μl injection volume at 327 nm absorbance. The total runtime was 25 min. The gamma-oryzanol component consists of the total peak area of cycloartenyl ferulate, 24-methylene cycloartenyl ferulate, campesteryl ferulate, and sitosteryl ferulate compared to the gamma-oryzanol standard series (0–0.5 μg/ml).
2.8.3 Analysis of phytosterols
The analysis was done in reference to Khatoon et al. (2010) with modifications. Around 20 μl samples were injected into the HPLC Shimadzu LC-20AD (Japan) with a column from Shim-pack VP-ODS 250 mm × 4.6 mm (Japan). The detector was a UV/VIS SPD-20A (Japan) with 206 nm absorbance. An isocratic mobile phase of methanol/bidistilled water (99:1, v/v) with a 0.5 ml/min flow rate was applied. Phytosterols were determined using campesterol, stigmasterol, and β-sitosterol standards by comparing their area under the curve. Campesterol, stigmasterol, and β-sitosterol retention times were around 31, 32, and 34 mins, respectively.
2.8.4 Analysis of vitamin E
The analysis was following Pokkanta et al. (2019) with modifications. About 20 μl was injected into the HPLC Shimadzu LC-20AD (Japan) (the column type was the same as stated before). The detector used was UV/VIS SPD-20A (Japan) with 295 nm absorbance. Methanol/bidistilled water (95:5, v/v) was applied as the isocratic mobile phase with a 0.5 ml/min flow rate. The determination of vitamin E was using α-tocopherol and β-, δ-, γ-tocotrienols standards by comparing their area under the curve. The retention times of α-tocopherol and β-, δ-, γ-tocotrienols were around 74, 36, 30, and 37 mins.
2.9 Statistical analysis
Data were analyzed using Minitab 20. The Kolmogorov–Smirnov test checked the normality of the data. One-way ANOVA was performed to identify significant differences among the three rice bran oils. Tukey’s post-hoc test for multiple comparisons using the value of p < 0.05. The data are the results of three or two repetitions and are expressed as the mean ± SD.
3 Results
3.1 Yield, recovery, and oxidative stability of RBO using modified TPP extraction method
Table 1 provides oil yield and recovery data across extraction time and rice bran types. The oil yields from white, red, and black rice bran vary significantly with extraction time (1, 1.5, and 2 h). The highest yield is observed at 2 h of extraction time for all types of rice bran. BRB possesses the highest yield at 14.00%, followed by RRB (9.73%) and WRB (6.83%). The rice bran used in this study, as raw materials, have the fat content of 11.38% for WRB, 15.36% for RRB, and 17.15% for BRB to count oil recovery using modified TPP. The highest recovery was found in BRB, reached 81%, while the WRB produced the lowest oil recovery. The results are statistically different (p < 0.05).
Figure 1 displays the oxidative stability of RBO from different rice brans and extraction times during storage. Among three RBOs on day 0 of 2 hours of extraction time, white RBO shows the highest PV of 15.94 mEq O2/kg, followed by red RBO (14.47 mEq O2/kg) and black RBO (13.55 mEq O2/kg). RBO must meet the Codex standard for Named Vegetable Oils (CODEX STAN 210-1999) with a maximum peroxide value of 15 mEq/kg for cold-pressed or virgin oils. White RBO shows levels slightly higher than the Codex Stan maximum. All RBOs are unrefined crude oils. Therefore, a series of refining processes will reduce the PV, such as bleaching and deodorization. Mingyai et al. (2017) found refined RBO from pigmented bran had a PV of 0.77–2.24mEq/kg, below Codex thresholds. Similarly, the PV slightly above 15 supports reports of quick deterioration in un-stabilized rice bran; without enzyme inactivation, endogenous lipases hydrolyze triglycerides to FFA and promote oxidation.
The trend for all RBO is similar, as it is increasing until day 21 and is decreasing until day 28. This curve is typical of PV during storage (Matthäus, 2010). The highest PV value for each white, black, and red RBO is 45.13, 39.76, and 40.45 mEq O2/kg on day 21, respectively. Like the PV, white RBO has the highest p-AnV on day 0 of 2 hours of extraction, with a level of 5.50, followed by red RBO (4.13) and black RBO (4.02). Differing with PV, the increasing trend of p-AnV in RBO is consistent throughout storage time. The increase of t p-AnV is sharp on day 28, where the value ranges from 24-28 among rice bran types.
Furthermore, because PV and p-AnV alone do not describe the whole oil quality, total oxidation (TOTOX) combines PV and p-AnV to determine more comprehensive oil quality. The TOTOX value of black RBO is the lowest (on day 0 of 2 hours of extraction) with the level of 31.12, followed by red RBO (33.08) and white RBO (37.38). The value keeps increasing with the highest TOTOX value on day 28, ranging from 99-112 among rice bran types. It was concluded that the best extraction time based on oil yield was 2 hours.
Oil yield and recovery of white, red, and black rice brans based on extraction time
 |
Fig. 1 Peroxide, p-anisidine, and TOTOX value of rice bran oil from white (WRB), red (RRB), and black (BRB) rice bran obtained by 1-, 1.5-, and 2-hours of extraction. (x-axis labels consists of rice bran type abbreviations and the number represents days of incubation. Bars indicate standard deviations (n = 3). PV: peroxide value; p-AnV: p-anisidine value; TOTOX: total oxidation; mEq: milliequivalent; WRB: white rice bran; RRB: red rice bran; BRB: black rice bran). |
3.2 RBO color value
Color values of RBOs are shown in Table 2, and the appearance of RBO from the best extraction time is shown in Figure 2. The color value of RBOs extracted from WRB, RRB, and BRB shows distinct differences. As shown in Figure 2, the visual appearance of the RBO differs among rice bran types. These visual differences align with the colorimetric data. White RBO has the highest lightness value (L = 42.3), which indicates that it is the lightest in color, while black RBO has the lowest L value of 26.9. A similar trend is also observed in chroma value (C). White RBO has the highest C of 28.6, and BRB has the lowest C of 3.9, indicating more vibrant coloration of white RBO. The hue angle (h) also differs, with red RBO having the highest value of 68.5, which indicates a more yellowish color, while black RBO exhibits a dark reddish color from an h angle of 19.3. The differences (p < 0.05) in color could be attributed to the pigment content and the profiles of different types of rice bran.
Color value of white, red, and black rice bran oils from 2 hours of extraction
 |
Fig. 2 Rice bran oil appearance obtained from white (a), red (b), and black (c) rice bran from two hours of extraction. |
3.3 Free fatty acid and acid value
Figure 3 exhibits the data for free fatty acid (FFA) and acid value (AV) of RBOs from 2 hours of extraction time. These parameters are indicators of hydrolytic rancidity. Black RBO has the highest FFA content of 7.08%, followed by red RBO (6.52%) and white RBO (3.78%). The AV indicates a similar trend to the FFA value. The AV obtained is 7.52, 12.97, and 14.09 mg NaOH/g for white, red, and black RBOs, respectively. These findings suggest that black RBO may have more hydrolytic degradation. The higher FFA content in black and red RBOs indicates that these oils are probably more prone to hydrolysis.
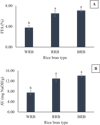 |
Fig. 3 Free fatty acid (A) and acid value (B) of rice bran oil from white, red, and black rice bran from two hours of extraction. (Bars indicate standard deviations (n = 3). Different letters for each measurement are significantly different at p < 0.05. FFA: free fatty acid; AV: acid value; WRB: white rice bran; RRB: red rice bran; BRB: black rice bran). |
3.4 Total phenolics, flavonoids, and anthocyanins content
Figure 4 displays the total phenolics (TPC), flavonoids (TFC), and anthocyanins content (TAC) of RBOs. RBO with the highest TPC and TAC belonged to the black RBO, followed by red and white RBOs. Although WRB oil has the lowest TPC, it significantly (p < 0.05) has the highest TFC, and the lowest is black RBO. Two of the colored RBOs have anthocyanins, while these compounds are not detected in white RBOs. Black RBO has more anthocyanins than the red one.
 |
Fig. 4 Total phenolics (TPC) (A), flavonoids (TFC) (B), and anthocyanins (TAS) (C) content of rice bran oil from white, red, and black rice bran from two hours of extraction. (Bars indicate standard deviations (n = 3). Different letters for each measurement are significantly different at p < 0.05. TPC: total phenolics content; TFC: total flavonoids content; TAC: total anthocyanins content; GAE: gallic acid equivalent; QE: quercetin equivalent; C3G: cyanidin 3-glucoside; WRB: white rice bran; RRB: red rice bran; BRB: black rice bran). |
3.5 Fatty acid profile of RBO
The fatty acid profile of RBO from white, red, and black rice bran is shown in Table 3. The fatty acid profile shows that all RBOs contain saturated and unsaturated fatty acids, with significant differences among rice bran types (p < 0.05). Palmitate is the only detected saturated fatty acid in all samples, with the highest of 39.66% in black RBO, while white RBO has the lowest (37.87%). RBO from different colors of rice bran has higher unsaturated fatty acids, with the predominant being linoleic acid. White RBO has higher linoleic acid (38.83%) than black RBO (35.32%). Different fatty acid profiles of RBO with different rice bran colors will affect the physicochemical properties of the oils, such as the propensity to oxidation, density, melting points, and others.
Fatty acid profile of the oils from white, red, and black rice brans from two hours of extraction
3.6 Gamma-oryzanol, phytosterols, and tocols content of RBO
Table 4 displays the concentrations of γ-oryzanol comprising cycloartenyl ferulate, oryzanol C (24-methylene cycloartanyl ferulate), camperteryl ferulate, and sitosteryl ferulate. The phytosterols consist of campesterol, stigmasterol, and β-sitosterol. Meanwhile, vitamin E comprises of α-tocopherol, β-, δ-, and γ-tocotrienol.
All RBOs contain γ-oryzanol with the highest is found in the red RBO. According to Xu and Godber (1999), three major γ-oryzanol from crude RBO were 24-methylenecycloartanyl ferulate, cycloartenyl ferulate, and campesteryl ferulate. Table 4 shows BRB oil has the highest γ-oryzanol and the lowest is the oil from WRB. Oryzanol C (24-methylene cycloartanyl ferulate) is the predominant γ-oryzanol in all RBO samples.
Phytosterols are also detected in all RBOs, and red RBO shows the highest phytosterols, with a total phytosterol concentration almost twice that found in white RBO, which has the lowest phytosterol level. Campesterol, stigmasterol, and β-sitosterol are found in all RBOs, but the high variability of each phytosterol concentration among RBOs from different rice bran colors. The concentration of phytosterols is higher than that of γ-oryzanol and tocotrienols.
Rice bran is well-known as the source of vitamin E, mainly tocotrienols (Ahsan et al., 2015). Table 4 shows that no tocopherol is detected in all RBOs, and some tocotrienols are found. The concentration of total tocotrienols is 82.25 ± 5.71, 17.73 ± 0.39, and 257.32 ± 19.51 mg/100 g in white, red, and black RBOs, respectively. β- and δ tocotrienol are found in all RBOs, while γ-tocotrienol is only found in RBO from WRB. Black RBO has the highest tocotrienol,s and the lowest is RBO from RRB. The concentration of δ-tocotrienol is much higher than that of β-tocotrienol in all RBOs samples.
Bioactive compound of the oils from white, red, and black rice brans from two hours of extraction
4 Discussion
4.1 Yield, recovery, and oxidative stability
The increasing extraction time increases oil yield, although BRB slightly increases between 1.5 and 2 hours of extraction. Oliveira et al. (2012) exhibited an oil yield of 8.56 to 20.05% from white rice bran with varying ethanol extraction experimental conditions. Wongwaiwech et al. (2023) showed the oil yields of 4.2 to 26.0% for the white rice bran and 5.4 to 20.8% for the red rice bran extracted by three green technologies. The rice varieties, rice bran particle size, and extracting solvents affected the yield of oil extraction. The solvents for extraction were n-hexane, petroleum ether, and chloroform, for 120 min using a Soxhlet extractor. Chloroform with rice bran particle size of 150 µm resulted in the highest oil yield (Ajali and Emembolu, 2024).
At the same time, RBO extraction using the modified TPP method is more efficient for BRB than WRB and RRB. The oil recovery for BRB can reach 81%, while BRB and RRB only reached around 60-63%, indicating modified TPP’s effectiveness in obtaining BRB oil. The extraction time is one of the determinants of RBO yield. Wang et al. (2020) revealed that there was no need to extend extraction time more than 1 h, as it did not significantly improve the yield of RBO. The findings in this research display different phenomena. The longer extraction time, more than 1.5 h, shows a significant increase in RBO yield from WRB. The difference in the results compared to the previous study can be attributed to several factors, such as the type of solvent, temperature, and the use of ammonium sulphate salt.
Commercially, rice bran oil is extracted with hexane (Garofalo et al., 2021). Other alternative organic solvents for extraction have been evaluated by Ribas et al. (2023), and the results showed that the highest oil yield was found in ethanol solvent extraction (24.45%), followed by ethyl acetate (22.39%), and n-hexane (19.92%). The extraction was carried out at a boiling solvent temperature for 4 h at a rice bran to solvent ratio of 5:150 (w/v). Improvement of rice bran oil yield was performed by pretreatment of fermentation before hexane extraction with Saccharomyces cerevisiae with the yield of 24.15% and Aspergillus niger with the yield of 14.20% (Yunardi et al., 2020). The oil yield from this study was much lower than that reported by Ribas et al. (2023). The modified TPP extraction may not have been carried out under optimum conditions because this extraction was first used for rice bran oil, and our focus was not yet on optimizing the oil yield. This study is an initial study as part of our consecutive studies to separate macro constituents from rice bran with different colors.
Figure 1 shows the pattern of oxidation products generation which is similar for all rice bran types. PV increases to the 21-day storage and decreases on day 28. Meanwhile, the p-AnV increases slowly to day 21 and sharply increases at day 28. PV is an indicator of primary oxidation products that are easily decomposed into secondary oxidation products. The decrease in PV was accompanied by an increase in p-AnV as an indicator of secondary oxidation products from peroxide degradation. Perhaps on day 28, no more primary oxidation products were produced, and peroxide decomposition began to occur, resulting in a decrease in PV and an increase in p-AnV. This phenomenon was also explained by Kumari et al. (2014), who found that PV increased up to 15 days of fish oil emulsion storage and then decreased. The study by Rahmania et al. (2020) exhibited the fragmentation of lipid peroxide during thermal oxidation.
Increasing extraction time also increases the oxidative stability of all RBOs (Fig. 1). Longer extraction time presumably enhances the extractability of bioactive compounds from rice bran. Tocotrienols are potent antioxidants that scavenge free radicals by donating their phenolic hydrogen (Mendonça et al., 2022). γ-Oryzanol is the most potent antioxidant in rice bran, acting as a scavenger for free radical (Rungratanawanich et al., 2020). Phytosterols also exhibit antioxidant activity (Mohamad et al., 2019), by inhibiting the generation of various radicals such as hydroperoxides (El Omari et al., 2024). Increasing PV and p-AnV during storage might also relate to the degradation of antioxidant compounds in RBOs. Rattanathanan et al. (2022) reported a decrease in antioxidant contents of rice bran during storage, following zero- and first-order reaction kinetics.
RBO from BRB shows the best oxidative stability, followed by RRB oil. The lowest oxidative stability is RBO from white rice bran. Vargas et al. (2018) reported that ferulic acid was the most dominant phenolic compound in black and red rice bran, and black rice bran also contains anthocyanins. The study of Wongwaiwech et al. (2023) showed that crude red RBO had higher total phytosterols than white RBO. All those compounds are potent antioxidants that prolong the oxidative stability of pigmented RBOs. Accordingly, Table 4 shows that the lowest antioxidant compounds comprising γ-oryzanol, tocotrienols, and phytosterols are white RBO. The oxidative stability of black RBO is better than that of red RBO, although red RBO has the highest γ-oryzanol, a typical strong antioxidant of rice bran (Lisnawati et al., 2022) and much higher phytosterol levels, which also contribute to antioxidant activity. However, black RBO contains higher phenolics and anthocyanins, which also have a role as antioxidants. Possibly, synergism occurs in black RBO to have the best oxidative stability among RBO from different rice bran.
4.2 Color value
The color of RBOs is one parameter affecting human/consumer acceptance (Lakshmi, 2014). White RBO has the lightest appearance, which is also proved by the L value of 42.3, meanwhile, black RBO has the lowest lightness value. Although red and black RBOs seem to have a similar appearance (Fig. 2). However, red RBO is more yellowish than black RBO, which is a purplish-red color. The color of black RBO is supported by the anthocyanins content (Fig. 4), especially from cyanidin 3-glucoside (Sompong et al., 2011), which has a bright red color (Han and Xu, 2015). High flavonoids of white RBO contribute to the yellowish color that also in accordance to the data of yellowness (+b). The C value of this RBO indicates the deep yellow color. Meanwhile, the red RBO has higher lightness than that of the black one, due to lower phenolics, flavonoids, and anthocyanins (Figure 4). The depth of black RBO is lower than that of the red one, indicating that the color of black RBO is brighter due to the bright red color of cyanidin 3-glucoside. Based on the hue value, all RBOs indicate the color in quadrant 1 of the chromaticity diagram, with black RBO tending to be near quadrant IV, indicating reddish and purplish colors. The two other RBOs have hue values that tend to be yellowish for the white RBO and reddish for the red RBO.
4.3 Free fatty acid and acid value
Free fatty acid (FFA) and acid value (AV) are parameters for describing the hydrolytic state of the RBOs. The hydrolysis might occur because the presence of sufficient water and the residual native lipase activity from rice bran, indicated by high free fatty acid and acid value, exceeds the maximum level of acid value based on the Codex Standard of 0.5 mg KOH/g. This fatty acid can be removed by refining steps, mainly in neutralization or deodorization. Higher FFA and AV are observed in black and red RBOs than in white RBO (p < 0.05), although Rattanathanan et al. (2022) reported a higher lipase activity in white rice bran than in black one. Li et al. (2023) revealed that flavonoids, depending on their structure, can inhibit lipase activity. White RBO has the highest flavonoids, thus it might reduce the lipase hydrolytic activity in the white RBO, producing lower FFA and AV.
The FFA and AV in this study are in the range of 3.78-7.08% and 7.52-14.09 mg NaOH/g, respectively. According to Mingyai et al. (2017), which extracted RBO from Thai paddy, the FFA was found in the lower concentration of 2-6%, while AV was in the range of 4-12 mg NaOH/g using three extraction methods, including cold-press, solvent, and supercritical CO2 extractions. A study by Rattanathanan et al. (2022) showed that raw rice bran has high lipase activity, leading to antioxidant loss during storage, while stabilized bran suppresses lipase and preserves antioxidants content. The extraction with modified TPP involves mixing with water, which increases the susceptibility to oxidation. The extraction methods affect the degree of RBO hydrolysis (Punia et al., 2021). No pretreatment to inactivate lipase also plays a role in the high FFA and AV.
4.4 Total phenolics, flavonoids, and anthocyanins content
The phytochemical concentration of RBOs is represented by total phenolics (TPC), flavonoids (TFC), and anthocyanins content (TAC). The TPC content of RBOs ranged from 4.90 to 5.96 mg GAE/g. On the other hand, TFC and TAC ranged from 1.75 to 3.40 mg QE/g and 2.30-4.19 mg cyanidin 3 glucoside/g, respectively. Pigmented RBOs have higher TPC than non-colored RBOs (Fig. 4). Meanwhile, the highest TFC is observed in white RBO. Bani et al. (2024) revealed that pigmented rice had higher TPC and higher antioxidant activity.
The TPC from this study is lower than that reported by Mingyai et al. (2017) ranging from 6 to10 mg GAE/g oil, which might be affected by the extraction methods, rice varieties, paddy growing conditions, environment, and others. The pigmented RBOs contain higher phytochemical concentrations than those of white RBO. Bopitiya and Madhujith (2015) reported that methanolic extracts of colored rice bran, especially red rice bran, contain higher TPC than white rice bran, with the value of 2.63 mg GAE/g extract. Meanwhile, Junyusen et al. (2022) reported the TPC and TFC concentrations ranged from 3.79 to 6.57 mg GAE/g and 0.35 to 1.95 mg CE/g, respectively, depending on the extraction methods.
In contrast, WRB exhibited an unusually high total flavonoid content (TFC) of 340 mg QE/100 g, surpassing that of RRB and BRB oils. This unusual finding is likely due to the specific composition of WRB, which contains non-colored flavonoids such as flavonols and flavones in abundance. Notably, published studies on white rice bran have revealed considerable amounts of flavonols like rutin (quercetin-3-rutinoside), various quercetin derivatives, and myricetin, along with flavones including kaempferol glycosides and the methylated flavone tricin (Andriani et al., 2022; Chen et al., 2022). Tricin, a flavone unique to bran, constitutes the majority of flavonoids found in rice bran (Poulev et al., 2019). These colorless flavonoids produce a strong reaction in the AlCl₃-based flavonoid assay (quantified as quercetin equivalents), indicating that a WRB rich in quercetin, kaempferol, and tricin can easily result in a very high TFC.
Black and red RBOs have considerable amounts of anthocyanins, but no anthocyanins are detected in white RBO. Anthocyanins contribute generously to the color of rice bran and their corresponding RBO. Chen et al. (2024) explained that the anthocyanin composition of rice bran depends on the type of colored rice. However, there is a typical anthocyanin, such as cyanidin 3-glucoside, in the red, purple, and black rice brans. It seems that only part of the anthocyanins are soluble in the RBO, since the polarity of the anthocyanin compound might vary, depending on their structures. Some anthocyanins exhibit lower polarity and higher hydrophobicity (Sang et al., 2018), that might dissolve in RBO.
4.5 Fatty acid profile
In all RBOs, unsaturated fatty acids are higher than saturated ones, which only comprise palmitate (Tab. 3). Wisetkomolmat et al. (2022) reported the predominant palmitic acid in the various varieties of rice bran. Other saturated fatty acids found are C14:0-C24:0, with a much lower concentration than palmitate. This study only detected one saturated fatty acid, palmitate. Unsaturated fatty acids are dominated by linoleic acid; others are palmitooleate, oleate, and α-linolenate. Wisetkomolmat et al. (2022) and Amrinola et al. (2022) indicated that the predominant unsaturated fatty acid is oleate, followed by linoleate. Pigmented RBOs have lower unsaturated fatty acids than those of white RBO. This means that pigmented rice is slightly more stable to oxidation due to higher saturated fatty acids, besides the presence of antioxidant compounds.
4.6 Gamma-oryzanol, phytosterols, and vitamin E content
All bioactive compounds analyzed are found in unsaponifiable matters of RBO and fat-soluble properties. Table 4 shows the concentration of γ-oryzanol is 474=730 mg/100 g, phytosterols of 730-1433 mg/100 g, and tocotrienols are 17-257 mg/100 g. In comparison, Sawadikiat and Hongsprabhas (2014) reported that crude RBO had 1362–1376 mg/100 g phytosterol per 100 g, 1599–1666 mg/100 g γ-oryzanol.
Table 4 shows that four compounds of γ-oryzanol are detected in all RBO samples, in which three compounds (cycloartenyl ferulate, 24-methylene cycloartanyl ferulate, campesteryl ferulate) are the major typical of rice bran, with a significant antioxidant activity (Xu and Godber, 2001). γ-Oryzanol is composed mainly of esters of trans-ferulic acid with phytosterols and triterpenic alcohols (Lerma-García et al., 2009). In this study, two compounds are esterified with sitosterol and campesterol, and two compounds are bound to triterpenic alcohol.
The major γ-oryzanol in all RBOs is oryzanol C (24-methylene cycloartanyl ferulate) (Tab. 4), with the highest concentration found in black RBO and the lowest in white RBO. This compound had more vigorous anti-inflammation activity than cycloartenyl ferulate on the mice ear edema (Liu et al., 2013). Meanwhile, cycloartenyl ferulate, a second major γ-oryzanol in all RBOs, is an anti-allergic agent (Oka et al., 2010). The two phytosterol ferulate compounds might have hypolipidemic activity (He et al., 2024). The higher presence of γ-oryzanol compounds in pigmented RBO, mainly black RBO, with the highest concentration, makes the pigmented RBOs healthier. These compounds also improve RBO oxidative and vitamin E stability due to heat treatment (Ali et al., 2023).
The concentration of phytosterols in all RBOs is higher than that of γ-oryzanol and tocotrienols. The highest level is observed in red RBO, and the lowest is in white. Among phytosterols, campesterol is the most abundant in pigmented RBOs; meanwhile, in the white RBO, the predominant is stigmasterol. Campesterol, stigmasterol, and β-sitosterol concentrations ranged between 270–658, 79–368, and 91–509 mg/100 g, respectively. Phytosterols of crude and refined RBO were 848–1034 mg/100 g and 1362–1376 mg/100 g, respectively (Sawadikiat and Hongsprabhas, 2014), and 878–1143 mg/100 g (Mingyai et al., 2018). Wongwaiwech et al. (2023) reported that hexane extracted red RBO had higher content of various phytosterols than white RBO, with the levels of campesterol, stigmasterol, and β-sitosterol being 180, 240, and 736 mg/100 g, respectively. Meanwhile, hexane extracted white RBO had 72 mg/100 g campesterol, 132 mg/100 g stigmasterol, and 371 mg/100 g β-sitosterol. Factors affecting phytosterol levels in RBO were rice varieties, paddy growing conditions, environment, cultivation system, and extraction (Chew and Teng, 2023).
The vitamin E family comprises four tocotrienols (α, β, δ, γ) and tocopherols (α, β, δ, γ) (Jiang, 2014). Tocotrienols have superior anti-inflammatory and antioxidant properties over α-tocopherol (Peh et al., 2016). Table 4 shows that not all tocopherols and tocotrienols are detected in RBO samples. β- and δ-tocotrienols range from 1 to 22 and 15 to 252 mg/100 g, respectively, with total tocotrienols ranging from 17-257 mg/100 g. γ-Tocotrienol is only found in white RBO with a level of 13.02 mg/100 g. Tian et al. (2024) summarized the tocotrienol concentrations in RBO, in which γ-tocotrienol was 10.7-78.3 mg/100 g, and δ-tocotrienol was 0.5-3.86 mg/100 g. This study shows higher tocotrienol levels than those previously reported. The black RBO has the highest tocotrienol level, while the red RBO has the lowest. Tocotrienols contribute to the oxidative stability of RBO, in which the black RBO is the highest, and the lowest is the white RBO. The red RBO has the lowest tocotrienol level, but this oil contains high phytosterols and moderately γ-oryzanol that also contribute to inhibiting lipid oxidation.
5 Conclusions
This study highlights a modified TPP method for extracting RBO oil from white, red, and black rice bran, as a simultaneous method to separate macro components of rice bran. The RBO oxidative stability is affected by the presence of antioxidant bioactive compounds, with black RBO being the highest. The color of RBOs is affected by the bioactive compounds, mainly anthocyanins and flavonoids. RBO reveals high hydrolytic products, free fatty acids, with significant concentrations in pigmented RBOs. Dominated unsaturated fatty acids in RBOs lead to oxidation susceptibility, with the highest found in white RBO. Lower bioactive compounds and higher unsaturated fatty acids make white RBO the most susceptible to oxidation. Phytosterols, γ-oryzanol, and tocotrienols, besides phenolics, flavonoids, and anthocyanins, contribute to the oxidative stability and color of RBOs. This study provides insight into the superiority of pigmented RBOs over non-colored RBOs for food and health purposes and as food ingredients.
Acknowledgments
The authors greatly appreciate and thank the Directorate General of Higher Education, Research, and Technology, Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia for funding this research.
Funding
This research was funded through the Directorate General of Higher Education, Research, and Technology, Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia, for funding this research through the PMDSU Grant (Contract No. 00242.8/UN10.A050/B/PT 01.03.2/2024).
Data availability statement
The data supporting this study’s findings are included within the article. Additional raw data are available from the corresponding author (Teti Estiasih) upon reasonable request.
Conflicts of interest
The authors declare no conflict of interest.
Author contribution statement
Miftahurrahmi: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing − original draft. Teti Estiasih: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing − original draft, Writing − review & editing. Tunjung Mahatmanto: Supervision. Ahmad Zaki Mubarok: Supervision.
References
- Ahsan H, Ahad A, Siddiqui WA. 2015. A review of characterization of tocotrienols from plant oils and foods. J Chem Biol 8 (2): 45–59. [Google Scholar]
- Ajali JJ, Emembolu LN. 2024. Comparative analysis of solvent extraction of rice bran oil from various sources. Eur J Sustain Dev Res 8 (1): 1–6. [Google Scholar]
- Ali MA, Chew SC, Majid FAA. 2023. Contribution of endogenous minor components in the oxidative stability of rice bran oil. J Food Meas Charact 17 (1): 187–210. [Google Scholar]
- Amrinola W, Sitanggang AB, Kusnandar F, Budijanto S. 2022. Characterization of pigmented and non-pigmented flakes glutinous rice (ampiang) on chemical compositions, free fatty acids compositions, amino acids compositions, dietary fiber content, and antioxidant properties. Food Sci Technol 42: 1–7. [Google Scholar]
- Andriani R, Subroto T, Ishmayana S, Kurnia D. 2022. Enhancement methods of antioxidant capacity in rice bran: a review. Foods 11 (19): 1–25. [Google Scholar]
- Balachandran C, Mayamol PN, Thomas S, Sukumar D, Sundaresan A, Arumughan C. 2008. An ecofriendly approach to process rice bran for high quality rice bran oil using supercritical carbon dioxide for nutraceutical applications. Bioresour Technol 99 (8): 2905–2912. [Google Scholar]
- Bani C, Cappa C, Restani P, Sala M, Colombo F, Mercogliano F, Di Lorenzo C. 2024. Physicochemical and nutritional quality of pigmented rice and bran: influence of milling and cooking. Lwt 208: 1–12. [Google Scholar]
- Bhat FM, Sommano SR, Riar CS, Seesuriyachan P, Chaiyaso T, Prom-U-thai C. 2020. Status of bioactive compounds from bran of pigmented traditional rice varieties and their scope in production of medicinal food with nutraceutical importance. Agronomy 10 (1817): 1–15. [Google Scholar]
- Bopitiya D, Madhujith T. 2015. Antioxidant potential of rice bran oil prepared from red and white rice. Trop Agric Res 26 (1): 1–11. [Google Scholar]
- Chen T, Xie L, Wang G, Jiao J, Zhao J, Yu Q, Chen Y, Shen M, Wen H, Ou X, et al. 2024. Anthocyanins-natural pigment of colored rice bran: Composition and biological activities. Food Res Int 175: 1–10. [Google Scholar]
- Chen X, Yang Y, Yang X, Zhu G, Lu X, Jia F, Diao B, Yu S, Ali A, Zhang H, et al. 2022. Investigation of flavonoid components and their associated antioxidant capacity in different pigmented rice varieties. Food Res Int 161: 1–12. [Google Scholar]
- Chew KW, Ling TC, Show PL. 2018. Recent developments and applications of three-phase partitioning for the recovery of proteins. Sep Purif Rev 00: 1–13. [Google Scholar]
- Chew SC, Teng SK. 2023. Bioactive phytochemicals from rice bran oil processing by-products. Di dalam: In: Reference Series in Phytochemistry. hlm, pp. 65–103. [Google Scholar]
- CODEX Alimentarius, International Food Standard. n.a. Standard For Named Vegetable Oils. CODEX Stan 210–1999. Adopted in 1999. Revision: 2001, 2003, 2009. Amendment: 2005, 2011, 2013 and 2015. Food and Agriculture Organization and World Health Organization, United Nations. [Google Scholar]
- Deepika D, Vegneshwaran V, Julia P, Sukhinder K, Sheila T, Heather M, Wade M. 2014. Investigation on oil extraction methods and its influence on omega-3 content from cultured salmon. J Food Process Technol 5 (12): 1–13. [Google Scholar]
- Franklin EC, Haq M, Roy VC, Park JS, Chun BS. 2020. Supercritical CO2 extraction and quality comparison of lipids from Yellowtail fish (Seriola quinqueradiata) waste in different conditions. J Food Process Preserv 44 (11): 1–12. [Google Scholar]
- Friedman M. 2013. Rice brans, rice bran oils, and rice hulls: composition, food and industrial uses, and bioactivities in humans, animals, and cells. J Agric Food Chem 61 (45): 10626–10641. [Google Scholar]
- Garofalo SF, Tommasi T, Fino D. 2021. A short review of green extraction technologies for rice bran oil. Biomass Convers Biorefinery 11 (2): 569–587. [Google Scholar]
- Han FL, Xu Y. 2015. Effect of the structure of seven anthocyanins on self-association and colour in an aqueous alcohol solution. South African J Enol Vitic 36 (1): 105–116. [Google Scholar]
- He W Sen Zhao L, Yang H, Rui J, Li J, Chen ZY. 2024. Novel synthesis of phytosterol ferulate using acidic ionic liquids as a catalyst and its hypolipidemic activity. J Agric Food Chem 72 (4): 2309–2320. [Google Scholar]
- Jiang Q. 2014. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med 72: 76–90. [Google Scholar]
- Junyusen T, Chatchavanthatri N, Liplap P, Junyusen P, Phan VM, Nawong S. 2022. Effects of extraction processes on the oxidative stability, bioactive phytochemicals, and antioxidant activity of crude rice bran oil. Foods 11 (1143): 1–19. [Google Scholar]
- Khan MAI, Ueno K, Horimoto S, Komai F, Tanaka K, Ono Y. 2009. Physicochemical, including spectroscopic, and biological analyses during composting of green tea waste and rice bran. Biol Fertil Soils 45 (3): 305–313. [Google Scholar]
- Khatoon S, Rajan RGR, Krishna AGG. 2010. Physicochemical characteristics and composition of indian soybean oil deodorizer distillate and the recovery of phytosterols. JAOCS, J Am Oil Chem Soc 87 (3): 321–326. [Google Scholar]
- Kumari JA, Venkateshwarlu G, Choukse M, Anandan R. 2014. Effect of essential oil and aqueous extract of ginger (Zingiber officinale) on oxidative stability of fish oil-in-water emulsion. J Food Process Technol 06 (01): 6–11. [Google Scholar]
- Lai OM, Jacoby JJ, Leong WF, Lai WT. 2019. Nutritional studies of rice bran oil. Di dalam: In: Rice Bran and Rice Bran Oil: Chemistry, Processing and Utilization. hlm, pp. 19–54. [Google Scholar]
- Lakshmi C. 2014. Food coloring: The natural way. Res J Chem Sci 4 (2): 2231–606. [Google Scholar]
- Lerma-García MJ, Herrero-Martínez JM, Simó-Alfonso EF, Mendonça CRB, Ramis-Ramos G. 2009. Composition, industrial processing and applications of rice bran γ-oryzanol. Food Chem 115 (2): 389–404. [Google Scholar]
- Li MM, Chen YT, Ruan JC, Wang WJ, Chen JG, Zhang QF. 2023. Structure-activity relationship of dietary flavonoids on pancreatic lipase. Curr Res Food Sci 6: 1–8. [Google Scholar]
- Lisnawati L, Poeranto S, Endharti AT, Santoso MIE. 2022. Antioxidant and anti-inflammatory activity of γ-oryzanol compared to rice bran oil to repair ovarian histological structure from one push transfluthrin exposure effect. Open Access Maced J Med Sci 10(B): 1–12. [Google Scholar]
- Liu M, Yang F, Shi H, Akoh CC, Yu L. 2013. Preparative separation of triterpene alcohol ferulates from rice bran oil using a high performance counter-current chromatography. Food Chem 139 (1-4): 919–924. [Google Scholar]
- Liu Z, Yu D, Li L, Liu X, Zhang H, Sun W, Lin CC, Chen J, Chen Z, Wang W, et al. 2019. Three-phase partitioning for the extraction and purification of polysaccharides from the immunomodulatory medicinal mushroom Inonotus obliquus. Molecules 24 (403): 1–14. [Google Scholar]
- Manzoor A, Pandey VK, Dar AH, Fayaz U, Dash KK, Shams R, Ahmad S, Bashir I, Fayaz J, Singh P, et al 2023. Rice bran: nutritional, phytochemical, and pharmacological profile and its contribution to human health promotion. Food Chem Adv 2: 1–12. [Google Scholar]
- Marinova D, Ribarova F, Atanassova M. 2005. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J Univ Chem Technol Metall 40 (3): 255–260. [Google Scholar]
- Matthäus B. 2010. Oxidation of edible oils. Di dalam: In: Oxidation in Foods and Beverages and Antioxidant Applications. hlm, pp.183–238. [Google Scholar]
- Mehta BM, Darji VB, Aparnathi KD. 2015. Comparison of five analytical methods for the determination of peroxide value in oxidized ghee. Food Chem 185: 449–453. [Google Scholar]
- Mendonça J da S, Guimarães R de CA, Zorgetto-Pinheiro VA, Fernandes CD Pietro, Marcelino G, Bogo D, Freitas K de C, Hiane PA, Melo ES de P, Vilela MLB, et al 2022. Natural antioxidant evaluation: a review of detection methods. Molecules 27 (11): 1–37. [Google Scholar]
- Mingyai S, Kettawan A, Srikaeo K, Singanusong R. 2017. Physicochemical and antioxidant properties of rice bran oils produced from colored rice using different extraction methods. J Oleo Sci 66 (6): 565–572. [Google Scholar]
- Mingyai S, Srikaeo K, Kettawan A, Singanusong R, Nakagawa K, Kimura F, Ito J. 2018. Effects of extraction methods on phytochemicals of rice bran oils produced from colored rice. J Oleo Sci 67 (2): 135–142. [Google Scholar]
- Mo S, Dong L, Hurst WJ, Van Breemen RB. 2013. Quantitative analysis of phytosterols in edible oils using APCI liquid chromatography-tandem mass spectrometry. Lipids 48 (9): 949–956. [Google Scholar]
- Mohamad R, Agus BAP, Hussain N. 2019. Changes of phytosterols, rheology, antioxidant activity and emulsion stability of salad dressing with cocoa butter during storage. Food Technol Biotechnol 57 (1): 59–67. [Google Scholar]
- Morales-Ramos JA, Rojas MG, Kelstrup HC, Emery V. 2020. Self-selection of agricultural by-products and food ingredients by Tenebrio molitor (Coleoptera: Tenebrionidae) and impact on food utilization and nutrient intake. Insects 11 (12): 1–15. [Google Scholar]
- Nidhishree AS, Menezes RA, Venkatachalam H, Bhat KS. 2024. Rice bran as a sustainable source for value added materials: an overview. 4th vol. Springer International Publishing. [Google Scholar]
- Oka T, Fujimoto M, Nagasaka R, Ushio H, Hori M, Ozaki H. 2010. Cycloartenyl ferulate, a component of rice bran oil-derived γ-oryzanol, attenuates mast cell degranulation. Phytomedicine 17 (2): 152–156. [Google Scholar]
- Oliveira R, Oliveira V, Aracava KK, Rodrigues CEDC. 2012. Effects of the extraction conditions on the yield and composition of rice bran oil extracted with ethanol − a response surface approach. Food Bioprod Process 90: 22–31. [Google Scholar]
- El Omari N, Bakrim S, Khalid A, Abdalla AN, Iesa MAM, El Kadri K, Tang SY, Goh BH, Bouyahya A. 2024. Unveiling the molecular mechanisms: dietary phytosterols as guardians against cardiovascular diseases. Nat Products Bioprospect 14 (1): 1–29. [Google Scholar]
- Pal YP, Pratap AP. 2017. Rice bran oil: A versatile source for edible and industrial applications. J Oleo Sci 66 (6): 551–556. [Google Scholar]
- Park PW, Goins RE. 1994. In situ preparation of fatty acid methyl esters for analysis of fatty acid composition in foods. J Food Sci 59 (6): 1262–1266. [Google Scholar]
- Peh HY, Tan WSD, Liao W, Wong WSF. 2016. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol Ther 162: 152–169. [Google Scholar]
- Peng Y, Kyriakopoulou K, Ndiaye M, Bianeis M, Keppler JK, van der Goot AJ. 2021. Characteristics of soy protein prepared using an aqueous ethanol washing process. Foods 10 (9): 1–22. [Google Scholar]
- Pokkanta P, Sookwong P, Tanang M, Setchaiyan S, Boontakham P, Mahatheeranont S. 2019. Simultaneous determination of tocols, γ-oryzanols, phytosterols, squalene, cholecalciferol and phylloquinone in rice bran and vegetable oil samples. Food Chem 271: 630–638. [Google Scholar]
- Poulev A, Heckman JR, Raskin I, Belanger FC. 2019. Tricin levels and expression of flavonoid biosynthetic genes in developing grains of purple and brown pericarp rice. PeerJ: 1–23. [Google Scholar]
- Punia S, Kumar M, Siroha AK, Purewal SS. 2021. Rice bran oil: Emerging trends in extraction, health benefit, and its industrial application. Rice Sci 28 (3): 217–232. [CrossRef] [Google Scholar]
- Rahmania H, Kato S, Sawada K, Hayashi C, Hashimoto H, Nakajima S, Otoki Y, Ito J, Nakagawa K. 2020. Revealing the thermal oxidation stability and its mechanism of rice bran oil. Sci Rep 10 (1): 1–11. [CrossRef] [PubMed] [Google Scholar]
- Rattanathanan Y, Kanha N, Osiriphun S, Rakariyatham K, Klangpetch W, Laokuldilok T. 2022. Changes in content of antioxidants and hydrolytic stability of black rice bran after heat- and enzymatic stabilizations and degradation kinetics during storage. J Food Process Preserv 46 (12): e16795. [Google Scholar]
- Ribas FBT, Gasparetto H, Salau NPG. 2023. Sustainable extraction of rice bran oil: Assessing renewable solvents, kinetics, and thermodynamics. Chem Eng Res Des 197: 342–354. [Google Scholar]
- Rungratanawanich W, Abate G, Uberti D. 2020. Pharmacological profile of γ-oryzanol: Its antioxidant mechanisms and its effects in age-related diseases. 2nd ed. Elsevier Inc. [Google Scholar]
- Sang J, Dang K Kai, Ma Q, Li B, Huang Y Ya, Li C Qin. 2018. Partition behaviors of different polar anthocyanins in aqueous two-phase systems and extraction of anthocyanins from Nitraria tangutorun Bobr. and Lycium ruthenicum Murr. Food Anal Methods 11 (4): 980–991. [Google Scholar]
- Sapwarobol S, Saphyakhajorn W, Astina J. 2021. Biological functions and activities of rice bran as a functional ingredient: A review. Nutr. Metab. Insigh. 5(14): 11786388211058559. [Google Scholar]
- Sawadikiat P, Hongsprabhas P. 2014. Phytosterols and γ-oryzanol in rice bran oils and distillates from physical refining process. Int J Food Sci Technol 49 (9): 2030–2036. [Google Scholar]
- Sharma GN, Dubey SK, Sati N, Sanadya J. 2011. Phytochemical screening and estimation of total phenolic content in aegle marmelos seeds. Int J Pharm Clin Res 3 (2): 27–29. [Google Scholar]
- Sompong R, Siebenhandl-Ehn S, Linsberger-Martin G, Berghofer E. 2011. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chem 124 (1): 132–140. [CrossRef] [Google Scholar]
- Tian X, Wang Xueyan, Fang M, Yu L, Ma F, Wang Xuefang, Zhang L, Li P. 2024. Nutrients in rice bran oil and their nutritional functions: a review. Crit Rev Food Sci Nutr: 1–19. [Google Scholar]
- Vargas CG, da Silva Junior JD, Rabelo TK, Moreira JCF, Gelain DP, Rodrigues E, Augusti PR, Rios A de O, Flôres SH. 2018. Bioactive compounds and protective effect of red and black rice brans extracts in human neuron-like cells (SH-SY5Y). Food Res Int 113: 57–64. [Google Scholar]
- Wang H, Geng H, Chen J, Wang X, Li D, Wang T, Yu D, Wang L. 2020. Three phase partitioning for simultaneous extraction of oil, protein and polysaccharide from rice bran. Innov Food Sci Emerg Technol 65: 1–10. [Google Scholar]
- Wisetkomolmat J, Arjin C, Satsook A, Seel-audom M, Ruksiriwanich W, Prom-u-Thai C, Sringarm K. 2022. Comparative analysis of nutritional components and phytochemical attributes of selected Thai rice bran. Front Nutr 9: 1–12. [Google Scholar]
- Wongwaiwech D, Kamchonemenukool S, Ho CT, Li S, Majai N, Rungrat T, Sujipuli K, Pan MH, Weerawatanakorn M. 2023. Bioactives from crude rice bran oils extracted using green technology. Molecules 28 (6): 1–20. [Google Scholar]
- Wrolstad RE, Giusti MM. 2001. Characterization and measurement of anthocyanins by UV-Vis spectroscopy. Curr Protoc Food Anal Chem 00 (1): F1.2.1–F1.2.13. [Google Scholar]
- Xu Z, Godber J. 1999. Purification and identification of components of γ-oryzanol in rice bran oil. J Agric Food Chem 47 (7): 2724–2728. [Google Scholar]
- Xu Z, Godber J. 2001. Antioxidant activities of major components of γ-oryzanol from rice bran using a linoleic acid model. J Am Oil Chem Soc 78 (6): 645–649. [Google Scholar]
- Yunardi Y, Meilina H, Fathanah U, Mahadina R, Rinaldi A, Jauharlina J. 2020. Enhancing rice bran oil yield through solid state fermentation pretreatment with fungi. Rasayan J Chem 13 (3): 1537–1543. [Google Scholar]
Cite this article as: Miftahurrahmi, Estiasih T, Mahatmanto T, Mubarok AZ. 2025. Bioactive compounds and oxidative stability of the oils from white, red, and black rice brans obtained by modified three-phase partitioning. OCL 32: 23. https://doi.org/10.1051/ocl/2025018.
All Tables
Oil yield and recovery of white, red, and black rice brans based on extraction time
Fatty acid profile of the oils from white, red, and black rice brans from two hours of extraction
Bioactive compound of the oils from white, red, and black rice brans from two hours of extraction
All Figures
 |
Fig. 1 Peroxide, p-anisidine, and TOTOX value of rice bran oil from white (WRB), red (RRB), and black (BRB) rice bran obtained by 1-, 1.5-, and 2-hours of extraction. (x-axis labels consists of rice bran type abbreviations and the number represents days of incubation. Bars indicate standard deviations (n = 3). PV: peroxide value; p-AnV: p-anisidine value; TOTOX: total oxidation; mEq: milliequivalent; WRB: white rice bran; RRB: red rice bran; BRB: black rice bran). |
| In the text | |
 |
Fig. 2 Rice bran oil appearance obtained from white (a), red (b), and black (c) rice bran from two hours of extraction. |
| In the text | |
 |
Fig. 3 Free fatty acid (A) and acid value (B) of rice bran oil from white, red, and black rice bran from two hours of extraction. (Bars indicate standard deviations (n = 3). Different letters for each measurement are significantly different at p < 0.05. FFA: free fatty acid; AV: acid value; WRB: white rice bran; RRB: red rice bran; BRB: black rice bran). |
| In the text | |
 |
Fig. 4 Total phenolics (TPC) (A), flavonoids (TFC) (B), and anthocyanins (TAS) (C) content of rice bran oil from white, red, and black rice bran from two hours of extraction. (Bars indicate standard deviations (n = 3). Different letters for each measurement are significantly different at p < 0.05. TPC: total phenolics content; TFC: total flavonoids content; TAC: total anthocyanins content; GAE: gallic acid equivalent; QE: quercetin equivalent; C3G: cyanidin 3-glucoside; WRB: white rice bran; RRB: red rice bran; BRB: black rice bran). |
| In the text | |
Les statistiques affichées correspondent au cumul d'une part des vues des résumés de l'article et d'autre part des vues et téléchargements de l'article plein-texte (PDF, Full-HTML, ePub... selon les formats disponibles) sur la platefome Vision4Press.
Les statistiques sont disponibles avec un délai de 48 à 96 heures et sont mises à jour quotidiennement en semaine.
Le chargement des statistiques peut être long.




