| Numéro |
OCL
Volume 31, 2024
Lipids from aquatic environments / Lipides issus des milieux aquatiques
|
|
|---|---|---|
| Numéro d'article | 23 | |
| Nombre de pages | 13 | |
| DOI | https://doi.org/10.1051/ocl/2024023 | |
| Publié en ligne | 5 novembre 2024 | |
Research Article
The yellow mullet fish oil from the Banc d’Arguin Imrâguens in Mauritania: an example of polyunsaturated fatty acids transfer from diatoms to the fish within the alimentary chain☆
L’huile de mulet jaune des Imrâguens du Banc d’Arguin en Mauritanie : un exemple de transfert des acides gras polyinsaturés des diatomées vers le poisson, au sein de la chaîne alimentaire
1
Unité de Chimie Moléculaire et Environnement, Université de Nouakchott, Mauritanie
2
Parc National du Banc d’Arguin, Chami, Mauritania
3
CIRAD, UMR QUALISUD, F34398 Montpellier, France
4
Qualisud, Univ Montpellier, Avignon Université, CIRAD, Institut Agro, IRD, Université de La Réunion, F34398 Montpellier, France
5
Aix-Marseille Univ, CEA, CNRS, Institute of Biosciences and Biotechnologies, BIAM Cadarache, 13108 Saint-Paul-lez-Durance, France
6
IRSN/PSN-RES/SAM/LETR, Centre de CADARACHE, Saint-Paul-Lez-Durance, France
7
Aix Marseille Univ, CNRS, UMR7281 Bioénergétique et Ingénierie des Protéines, 31 Chemin Joseph Aiguier, F-13009 Marseille, France
* Corresponding carriere@imm.cnrs.fr; hlaunay@imm.cnrs.fr; sidibounem@gmail.com
Received:
22
April
2024
Accepted:
19
September
2024
The Banc d’Arguin National Park (PNBA) in Mauritania is listed by the UNESCO World Heritage. It is characterized by an exceptionnal marine biodiversity with numerous endemic species and it provides a major site of reproduction for western Africa fish. The Imrâguens form fisherman communities established at Banc d’Arguin, who live upon fishing the yellow mullet (Mugil cephalus) during its migration and derived products. The fish oil produced by Imrâguens from mullet heads is rich in omega 3 polyunsaturated fatty acids (37.7 % of total fatty acids). The main fatty acid is eicosapentaenoic fatty acid (EPA ; 20.18 ± 0.01 %). This fatty acid is particularly abundant in diatoms, that contribute to 20- 30% of mullet feeding. The identification of 16:4n-1 also provide a good trophic marker for yellow mullet feeding on diatoms. The lipases potentially involved in the mobilization of these fatty acids in the course of digestion of diatoms were identified from the analysis of Mugil cephalus genome. Genes encoding a lipase homologous to gastric lipase and four lipases homologous to pancreatic carboxylester hydrolase or bile-salt stimulated lipase were identified. These later could be involved in the lipolysis of galactolipids, the main lipids present in diatom photosynthetic membranes which are rich in EPA. These data provide an added value to the traditional fishing practice of Imrâgens and highlight the nutritional value of the fish oil they produce.
Résumé
Le Banc d’Arguin est un Parc National Mauritanien (PNBA) inscrit au patrimoine mondial de l’UNESCO. Il présente une biodiversité marine exceptionnelle avec de nombreuses espèces endémiques et son écosystème constitue un site de reproduction majeur pour les poissons de l’Afrique de l’Ouest. Les Imrâguens sont des communautés maritimes établies au Banc d’Arguin qui pratiquent la pêche au mulet jaune (Mugil cephalus) lors de sa migration et vivent de sa transformation. L’analyse de l’huile extraite des têtes de mulet jaune par les Imrâguens montre une composition riche en acides gras polyinsaturés de la série oméga 3 (37,7 % des acides gras totaux). L’acide gras majoritaire est l’acide eicosapentaénoïque (EPA ; 20,18 ± 0,01 %). Cet acide gras est particulièrement abondant chez les diatomées, qui constituent 20 à 30% de l’alimentation de ce poisson. L’identification de 16:4n-1 constitue également un marqueur trophique de l’assimilation des acides gras des diatomées par le mullet jaune. Les lipases potentiellement responsables de la mobilisation de ces acides gras au cours de la digestion des lipides de diatomées ont été identifiées par une analyse du génome de Mugil cephalus. Celui-ci code pour une lipase homologue à la lipase gastrique et pour quatre lipases homologues à la carboxylester hydrolase pancréatique ou lipase dépendante des sels biliaires. Ce sont ces dernières enzymes qui pourraient être impliquées dans la lipolyse des galactolipides, constituants des membranes photosynthétiques des diatomées et riches en EPA. Ces résultats mettent en valeur la pêche artisanale pratiquée par les Imrâgens et la qualité nutritionnelle de l’huile de poisson qu’ils produisent à partir du Mulet jaune.
Key words: omega-3 fatty acids / galactolipase / grey mullet / fish oil / lipase / Mugil cephalus
Mots clés : acide gras oméga-3 / galactolipase / mulet gris / huile de poisson / lipase / Mugil cephalus
© M.V. Sidi Boune et al., Published by EDP Sciences, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Highlights
The fish oil from Yellow mullet is rich in eicosapentaenoic fatty acid and other PUFA like 16:4n-1 that reflect the large contribution of diatoms to its feeding habits. It suggests that PUFA from galactolipids of diatom photosynthetic membranes can be released by fish lipases upon digestion.
1 Introduction
The Banc d’Arguin National Park (PNBA) is located in the coastal zone of Mauritania, where the Sahara meets the Atlantic Ocean (Fig. 1). It is an exceptional spawning ground for West African marine species. Its shallow waters (5 m deep), representing 60% of its total area and extending 50 km from the coast, have been known for centuries by African fishermen and European explorers. They have sparked the interest of major colonial empires and are known for the wreck of the French frigate La Méduse and its famous raft immortalized by the Frenche painter Théodore Géricault. They are rich in biodiversity due to submerged marine meadows composed of three species of seagrasses, Zostera noltii, Cymodocea nodosa, and Halodule wrightii. The classification of Banc d’Arguin as a National Park in 1976 and its inscription as a UNESCO World Heritage Site (No. 506) owe much to Théodore Monod, who explored it as early as 1922 (Monod, 2001). With a maritime and continental area of 12,000 km2, it covers one-third of the Mauritanian coastline, from Minou Point to the town of Mamghar.
The populations of Mauritania, although primarily nomadic and attached to desert expanses, have also defended and benefited from this maritime wealth. The Imrâguens are maritime communities established on the Saharan coast long before the arrival of Arabs in the 15th century. Derived from the characteristic mixing of this region, they have developed a way of life linked to this ecosystem and based on fishing yellow mullets (Noray-Dardenne, 2006; Boulay, 2010; Bernardon and Vall, 2004; De Cenival and Monod, 1938). This fish is abundant during its spawning migration from the Gulf of Guinea to the West African coast. Upon capture, the fish is opened after cutting its head, cleaned, and dried by Imrâguen women who have perfected mullet processing methods passed down from mothers to daughters. These methods allow for its preservation and consumption throughout the year. The belief in its therapeutic virtues is widespread throughout West Africa, with annual cures or "guetna" and the consumption of yellow mullet in various forms: roasted (méchoui elhout), boiled (lemlouleb), or dried (tichtar). Its oil (dhên), consumed with dried fillets, is also renowned, as are its salted and dried eggs used to make bottarga (bayd elhout) (Fig. 2), rich in polyunsaturated fatty acids (Qiao et al., 2016; Bedhhi et al., 2004). Bottarga is a delicacy made from salted and cured fish roe pouch, typically of the grey mullet around the Mediterranean sea. The oil is extracted from boiled mullet heads in seawater, where it floats on the surface and is collected using a shell of Cymbium olla (Fig. 3).
The Yellow Mullet, also known as Flathead Grey Mullet (Mugil cephalus) is a coastal species of marine fish from the Mugilidae family (Fig. 4A). It is present on all coasts of temperate, tropical, and equatorial zones. It is a gregarious fish that lives on sandy or muddy bottoms, often at depths of less than 10 m. It feeds by suction of the upper layer of sediments, with sand grains contributing to food grinding in a gizzard inside its stomach. It mainly feeds on plankton (20–30% diatoms), dead plants, detritus from benthic organisms, and small fish. Microscopic examination of the gastric contents (cardiac zone; Fig. 4B) of yellow mullet shows (1) a large portion of quartz grains, mixed with (2) numerous benthic diatoms belonging to the genera Synedra, Nitzschia, Gyrosigma, Pleurosigma, Amphora and others, (3) aggregates consisting of fine particles of organic and mineral matter, and (4) debris from higher plants, probably marine seagrasses (Fig. 4B). Yellow mullet also feeds on epiphytic microalgae and epifauna covering seagrasses, cyanobacteria forming a mat on sediment surfaces, and microalgae present in foam at the water-air interface (Michaelis, 1993; Thomson, 1990; Whitfield et al., 2012). The identification of diatom silica frustules in gastric contents (Fig. 4B) supports the important contribution of diatoms to Yellow mullet feeding. Some are found with cracked shells and their plasma released as observed by microscopy (Michaelis, 1993).
A visit to Banc d’Arguin in December 2023 allowed us to observe the preparation of fish oil from yellow mullet heads by Imrâguens from the village of Mamghar near Cap Timiris. Here, we report the fatty acid composition of this oil and compare it to that of diatoms, which constitute the main food source for yellow mullet. We also searched for enzymes that could allow this fish to digest diatom lipids so that their fatty acids could be absorbed.
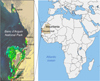 |
Fig. 1 Location of the Banc d’Arguin National Park in Mauritania. The left view is a Sentinel 2 map of the coastal marine biocenoses and terrestrial environments of the PNBA. Adapted from (Ewan Trégarot et al., 2019). |
 |
Fig. 2 Preparation of bottarga. The double pouch of yellow mullet eggs is manually removed when the fish is cut. It is then tied with a string, rinsed, salted for one hour, rinsed again, and left to dry in a ventilated area. |
 |
Fig. 3 Extraction of fish oil from yellow mullet heads. The cut heads are boiled in sea water in a large pot to separate the oil from the tissues, cartilage, and bones. After cooling, the oil floating on the surface is collected using a Cymbium shell and bottled. |
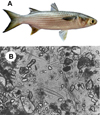 |
Fig. 4 The yellow mullet (Mugil cephalus) and its diet. (A) Photo of a yellow mullet (courtesy of J.D. Durand, Photographer). (B) Microscopic view of the stomach contents of the yellow mullet showing quartz grains, diatoms, and various aggregates. Reproduced with permission from (Michaelis, 1993). |
2 Materials and methods
2.1 Analysis of fish oil fatty acids by GC-FID
One single batch of fish oil from Mulet heads was obtained during our visit to PNBA. The fatty acids of fish oil triacylglycerols were converted into methyl esters (FAMEs) by transesterification in triplicate. In a 10–15 mL glass vial with a screw cap, 45 mg of oil was added to 2 mL of a 10% (v/v) solution of sodium methoxide in methanol. The reaction mixture was refluxed for 15 min, then 2 mL of acetyl chloride were added, and the mixture was refluxed again for 15 min. After cooling the vial, 3 mL of heptane were added to extract the FAMEs.
The analysis of FAMEs was first performed using an Agilent 8860GC gas chromatograph (Agilent Technologies, Les Ullis, France), equipped with a split injector (1/20 split ratio), a CP-Cil 88 Varian capillary column (50 m × 0.25 mm with a stationary phase of 0.2 µm thickness), and a flame ionization detector, using helium as the carrier gas (1 mL/min) and controlled by Openlab software (version B.01.18, 2019, Agilent Technologies, Les Ullis, France). For each analysis, the column temperature, initially set at 150 °C, was ramped up to 225 °C at a rate of 5 °C/min, then held at 225 °C for 10 min. The injector and detector temperatures were 250 and 270 °C, respectively. FAMEs were identified using external calibration with a mixture of known methyl esters, including EPA and DHA.
A second serie of fatty acid derivatization and ana lysis by GC-MS was performed in order to confirm fatty acid identification. One mL of a solution containing methanol with 5% (v/v) sulfuric acid was added to a drop of fish oil. Sample was incubated at 85 °C for 90 min in sealed glass tubes. After cooling down, FAMEs were extracted by adding 1 mL hexane and 1.5 mL of a solution water containing 0.9% (w/v) of NaCl. Sample was mixed and the organic phase was separated from the aqueous phase by centrifugation at 3,000g for 2 min. The hexane phase was recovered and 1 μL was injected onto on an Agilent 7890A gas chromatographer coupled to an Agilent 5975C mass spectrometer (simple quadrupole) and equipped with a Zebron 7HG-G007-11 (Phenomenex) polar capillary column (length 30 m, internal diameter 0.25 mm, and film thickness 0.25 μm). Helium carrier gas flow was 1 mL min−1. Oven temperature was programmed with an initial 1-min hold time at 60 °C, a first ramp from 60 to 150 °C at 10 °C min−1, then a second ramp from 150 to 260 °C at 5 °C min−1 and a final 5-min hold time at 260 °C. Samples were injected in splitless mode at 240 °C. The MS was run in full scan over 40 to 400 amu (electron impact ionization at 70 eV), and peaks were quantified based on the mass spectrum using NIST database.
For some case information on double bond positions, a fatty acid derivatization by 3-pyridylcarbinol to form 3-pyridylcarbinol (picolinyl) esters was used. One hundred μL of a solution of tert-butoxide in tetrahydrofuran were added to 200 μL of 3-pyridylcarbinol. After mixing, 50 μL of FAME in dichlorometane was added and the mixture was held at 40 °C for 1h in a glass tube. After cooling to room temperature, 1mL of water and 2 mL of hexane were added. After centrifugation 3-pyridyl carbinol derivatives were analyzed by UPLC-MS. Briefly, the lipid mixtures were first separated on a Kinetex™ (Kinetex, Atlanta, GA, USA) C18 2.1 × 150 mm 1.7 μm column (Phenomenex, Torrance, CA, USA) connected to a Vanquish UPLC system (Thermo Fisher, Waltham, MA, USA) coupled to a Thermo Orbitrap QExactive mass spectrometer. A binary gradient system of acetonitrile−water (60:40, v/v; eluent A) and isopropanol−acetonitrile (90:10, v/v; eluant B), both containing 10 mM ammonium formate, was performed by increasing eluant B from 7 to 97% in 26 min, followed by a 5-min hold, and then return to 7% of eluant B for another 6.9 min for column re-equilibration. The flow-rate was 0.3 mL.min−1. The column oven temperature was maintained at 45 °C. Qexactive mass spectrometer was used in positive mode with the following conditions: sheath gas at 50; sweep gas at 2; auxiliary gas at 15; spray voltage at 3kV; capillary temperature at 350 °C; heater temperature at 400 °C; S-lens RF at 45.3. Pyridyl carbinol esters identification was based on mass accuracy peaks and from the MS scan compared with theoretical masses. Peak of interest were collected several times in a glass tube. The collected fractions were evaporated and the 3-pyridyl carbinol esters were resuspended in 50 μL hexane and injected in GC-MS. Identification of the position of the doubles bonds was obtained by comparison with the archive of mass spectra available on LipidWeb (https://www.lipidmaps.org/resources/lipidweb/lipidweb_html/ms/pyrcarb.htm).
2.2 Bioinformatic analysis of the Mugil cephalus genome for digestive lipases
The genome of Mugil cephalus (FishBase ID: 785; NCBI Taxonomy ID: 48193) has been known since 2022 and is referenced in the NCBI database (PRJNA785274 (Zhao et al., 2022), PRJNA902967 and PRJNA675305 (Shekhar et al., 2022)). Sequences homologous to known digestive lipases, especially human enzymes, were searched using the BLAST program (https://blast.ncbi.nlm.nih.gov/Blast.cgi (Altschul et al., 1990)). Sequence alignments were obtained using the Clustal Omega program (Sievers et al., 2011).
2.3 Molecular modeling of digestive lipases
Three-dimensional molecular models of pancreatic carboxylesterases from Mugil cephalus were generated using the Phyre2 server (http://www.sbg.bio.ic.ac.uk/∼phyre2/html) (Kelley et al., 2015), their respective sequences (NCBI Reference Sequences XP_047426223.1, XP_047426224.1, XP_047426225.1, and XP_047448502.1), and the known crystallographic structure (PDB ID: 1JMY) of a truncated form of human pancreatic carboxylester hydrolase (HCEH), also known as bile salt-stimulated lipase (BSSL) (Moore et al., 2001). Molecular docking of a digalactosyldiacylglycerol (DGDG) molecule into the active site of HCEH was performed using the Autodock Vina program (Seeliger and de Groot, 2010; Trott and Olson, 2010), executed via PyMol software (PyMOL Molecular Graphics System, Version 1.3. Schrödinger, LLC; http://www.pymol.org/).
3 Results and discussion
3.1 Fatty acid composition of yellow mullet fish oil
Gas chromatography analysis of FAMEs derived from yellow mullet head oil (Tab. 1) reveals a typical composition of fish oils, rich in polyunsaturated omega 3 fatty acids (37.7% of total fatty acids). The most abundant fatty acid is eicosapentaenoic acid (EPA or C20:5 n-3; 20.18 ± 0.01%), followed by palmitic (C16:0; 16.31 ± 0.01%) and palmitoleic acids (C16:1; 13.12 ± 0.03%). Docosapentaenoic (DPA or C22:5 n-3; 5.98%), docosahexaenoic (DHA or C22:6 n-3; 6.66%), and oleic acids (C18:1; 6.78 ± 0.06%) are also present at high concentrations. The higher EPA content compared to DHA characterizes this oil, distinguishing it from the oil of carnivorous fish such as tuna and seabass, where DHA predominates over EPA (Tab. 2).
The composition of yellow mullet fish oil is similar to that of lipids from the marine diatom Phaeodactylum tricornutum, where EPA is also the major fatty acid (28.27 ± 0.47%; Tab. 1). It can thus be assumed that a significant portion of the EPA found in yellow mullet fish oil originates from the substantial presence of diatoms in its diet. In diatoms, polyunsaturated fatty acids including EPA are predominantly found in the major membrane lipids, galactolipids (Abida et al., 2015). They are poorly represented in triacylglycerols, except in cases of nitrogen or phosphorus deficiency (Abida et al., 2015). It is therefore reasonable to assume that yellow mullet possesses enzymes capable of digesting galactolipids and that the fatty acids released in the digestive tract by these enzymes can be absorbed and reused for triacylglycerol synthesis. This hypothesis is supported by the presence of hexatrienoic acid (C16:3) in fish oil (2.07 ± 0.01%; Table 1). This fatty acid is characteristic of galactolipids and it is found in plants (17.3 ± 0.5% in spinach MGDG (Amara et al., 2010)) as well as in diatoms (3.58 ± 0.02% in P. tricornutum (Table 1); 3.1 ± 1.4% in Asterionella formosa (Mekhalfi et al., 2014)). In zebrafish (Danio rerio) fed with spinach chloroplasts rich in C16:3 n-3, this fatty acid is found in the fish tissues (Gedi et al., 2019). However, 16:3 in diatoms differs by the positions of double bonds. It is 16:3 n-4 (Qiao et al., 2016).
It is worth noting that yellow mullet fish oil also contains fatty acids with 4 double bonds (16:4 and 18:4) representing more than 3% of total fatty acids each (Tab. 1). We observed that 16:4 from yellow mullet fish oil had a retention time that differed from 16:4 n-3 from the microalga Chlamydomonas reinhardtii used as a reference standard (Fig. 5, panels A and B).
In order to better describe 16:4 and identify the position of unsaturations, we performed a derivation of fatty acids into 3-pyridylcarbinol esters (or picolinyl esters), the mass spectrum of which contains fragment ions that provide this information. The principle is that under electron impact conditions, an electron is removed from the nitrogen of the pyridine ring and a hydrogen atom is abstracted from the alkyl chain to this electron-deficient site. This process produces a radical site that initiates alkyl chain cleavage. Hence, hydrogen atoms at any position of the alkyl chain can be removed with varying probabilities, depending on the fatty acid structure (Fig. 6A). In fine, the masses and relative abundances of the various ions produced in the mass spectrometer reflect the structure of the alkyl chain (Harvey, 1984, 1998). After separation by UPLC, the MS analysis of the 3-pyridylcarbinol-derivatized 16:4 from yellow mullet fish oil and Chlamydomonas reinhardtii (Fig. 5, panels C and D) confirmed the presence of double bonds at positions 6,9,12 and 15 in 16:4 from yellow mullet fish oil (Fig. 6B). We thus showed the presence of 16:4n-1, which is a good trophic marker of yellow mullet feeding on diatoms. This fatty acid is found for instance in the diatom Phaeodactylum tricornutum (Tab. 1).
We compared the fatty acid composition of the yellow mullet fish oil with that of the bottarga prepared from the same fish (Rosa et al., 2016). The latter is richer in DHA than EPA (Tab. 1) and more similar to the oil of tuna, bonito, and seabass (Tab. 2). This suggests that yellow mullet can convert some of the absorbed EPA into DHA and store it in its eggs. Fish eggs are known to be rich in DHA, especially in phospholipids that play a role in embryonic development (Sargent, 1995). There is selective incorporation of DHA into these lipids, which can come from dietary fatty acids (Bochert et al., 2023; Parma et al., 2015), mobilization of reserve lipids, or de novo synthesis (Wiegand, 1996).
From the known genome of M. cephalus, we searched for the presence of genes encoding fatty acid desaturases. In herbivorous fish, two fatty acid desaturase genes (fad1 and fad2) are found. Fad1 has a bifunctional Δ6-Δ5 Fad activity, while Fad2 has a bifunctional Δ4-Δ5 Fad activity and can convert DPA (22: 5n- 3) into DHA (22: 6n- 3) (Li et al., 2010). Fad2 from the herbivorous fish Siganus canaliculatus was shown to be a Δ4 fatty acid desaturase (Genebank accession number: GU594278.1) after expression in the yeast (Li et al., 2010). A BLAST search for fad homologs in Mugil cephalus reveals the presence of two fads2 transcript variants X1 and X2. According to NCBI Protein database, fads2 transcript variant X1 (XM_047595676.1) is annotated as Mugil cephalus acyl-CoA 6-desaturase isoform X1 (NCBI Reference Sequence: XP_047451632.1), while variant X2 (XM_047595677.1) is annotated as Mugil cephalus acyl-CoA 6-desaturase isoform X2 (NCBI Reference Sequence: XP_047451633.1). Our search also led to NCBI Reference Sequence XP_047466081.1, annotated as fatty acid desaturase 6 and related to another genome sequencing project.
A phylogenetic analysis of Fad1 and Fad2 with a variety of Fads of diverse species showed that marine teleost fish desaturases are most closely related to Δ6 Fads, and more distantly from lower eukaryotes Δ4 and Δ5 Fads. Mugil cephalus desaturases share 59.1% identity to human Δ6-FADS and they are thus annotated as Δ6 Fads in NCBI protein database. Both X1 and X2 isoforms shares 75% identity with Fad2 from Siganus canaliculatus (Genebank accession number: GU594278.1) that was shown to be a Δ4 fatty acid desaturase. However, they do not contain the YXXN domain responsible for the Δ4 desaturase function (Oboh et al., 2017). Therefore, we cannot confirm that yellow mullet possesses the enzymatic machinery to convert EPA into DHA.
We also found a fatty acid elongase (ELOVL; NCBI Reference Sequence: XP_047445583.1) with 41.44% identity to human ELOVL2.
Fatty acid composition of Yellow Mullet fish oil, bottarga (from the same fish), and lipids of the model marine diatom, Phaeodactylum tricornutum.
Composition in EPA, DPA and DHA of various fish oils.
 |
Fig. 5 Comparison of fatty acids from yellow mullet fish oil and total lipids from the microalga Chlamydomonas reinhardtii. Panel A and B: FAME separation by GC-MS using a polar Zebron 7HG-G007-11 capillary column. 16:4 from yellow mullet fish oil and Chlamydomonas reinhardtii show different retention times. Panel C and D: MS analysis on Thermo Orbitrap QExactive of 3-pyridinyl carbinol derivatives from yellow mullet fish oil and Chlamydomonas reinhardtii 16:4 after their separation by UPLC. The FAMEs from Chlamydomonas reinhardtii were produced from the total lipid extract of a culture performed as previously described (Gerard et al., 2022). |
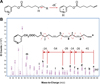 |
Fig. 6 Mass spectrometry analysis on Orbitrap Q Exactive of 3-pyridiylcarbinol derivative from 16:4 n-1 isolated from yellow mullet fish oil. (A) Principle of fragmentation based on random proton abstraction on the hydrocarbon chain of 3-pyridylcarbinol fatty acid derivatives according to (Harvey, 1984). (B) Mass spectrum of 3 pyridylcarbinol 6,9,12,15-hexadecatetraenotate showing the various fragments obtained upon electronic impact. |
3.2 Identification of Yellow Mullet Digestive Lipases
We found a single article reporting the partial purification and characterization of a yellow mullet lipase (Aryee et al., 2007). The specific activities measured with p-nitrophenyl esters (p-NP) are very low, and no activity on a natural lipase substrate (triacylglycerols) is reported. The activity on p-NP esters and the dependence of enzymatic activity on primary bile salts (higher with 3α,7α-dihydroxylated bile salts) suggest that it is a bile salt-dependent lipase (BSSL) or a carboxylesterase (CEH) rather than a classical pancreatic lipase (Lombardo et al., 1980; Lombardo and Guy, 1980).
Translation and BLAST analysis of the assembled genome PRJNA675305 of M. cephalus reveals the presence of a sequence (NCBI Reference Sequence: XP_047464368.1) homologous to that of human gastric lipase (56.35% identity; Bodmer et al., 1987). M. cephalus thus possesses a so-called preduodenal lipase (Moreau et al., 1988), without knowing yet in which tissues or organs its gene is expressed. It can thus be assumed at this stage that the digestion of triacylglycerols in yellow mullet may be partly carried out by this enzyme, although to date no fish gastric lipase has been purified and characterized. Furthermore, gastric lipase is not active on galactolipids (Wattanakul et al., 2019; Kergomard et al., 2022).
No homologous sequence to that of colipase-dependent classical pancreatic lipase is found in M. cephalus., what it is common in teleost bony fish representing 99.8% of current fish species (Tang et al., 2022), These fish and M. cephalus. have a diffuse pancreas unlike cartilaginous fish (shark, ray), which have a well-defined pancreas (Youson et al., 2006) and colipase, the protein cofactor of classical pancreatic lipase (Sternby et al., 1984; Ben Bacha et al., 2011). We did not find any gene homologous to colipase in M. cephalus, indicating the absence of the classical pancreatic lipase-colipase system. Similarly, no homologous sequence to pancreatic lipase-related protein 2 (PLRP2), an enzyme with galactolipase activity (Andersson et al., 1995; Sahaka et al., 2020) was found.
However, M. cephalus genome codes for a member of the pancreatic lipase gene family (NCBI Reference Sequence: XP_047458179.1) with greater homology to pancreatic lipase-related protein 1 (PLRP1) (Carrière et al., 1998). This protein identified and characterized in various mammal species lacks enzymatic activity and is usually characterized by two mutations (Ala178Val and Ala180Pro) compared to classical pancreatic lipase (Roussel et al., 1998). In M. cephalus, only the Ala178Val mutation is present. However, this mutation is sufficient to introduce steric hindrance that prevents the correct binding of a substrate at the active site (Roussel et al., 1998; Crenon et al., 1998). The physiological role of PLRP1 remains unknown, and it is surprising to find this inactive protein (if its gene is expressed) in a species lacking homologous genes encoding active lipases such as colipase-dependent classical pancreatic lipase and PLRP2. This situation has also been observed in cod (Gadus morhua (Saele et al., 2010)) and Japanese sea bream (Pagrus major (Oku et al., 2006)), without any proposed physiological role for this inactive protein (Ala178Val mutation). In sea bream, expression of the PLRP1 gene has been detected in adipose tissue and the hepatopancreas. This expression is not influenced by fasting or refeeding (Oku et al., 2006).
Finally, we found four genes (XP_047426223.1, XP_047426224.1, XP_047426225.1, and XP_047448502.1) homologous to human pancreatic BSSL or CEH (NP_001798.3). In literature and databases, this carboxylesterase (EC 3.1.1.1) with multiple lipid substrates appears under various names and abbreviations: CEL for carboxyl ester lipase, BSAL for bile-salt activated lipase, BAL for bile-activated lipase, or cholesterol esterase (Rudd and Brockman, 1984). We prefer the designation carboxylic ester hydrolase (CEH), which encompasses all enzymatic activities that this nonspecific enzyme may exert on various esters, as the term lipase is by definition associated with triacylglycerol hydrolases (EC 3.1.1.3). The four proteins homologous to CEH found in M. cephalus are named McCEH1 (XP_047426223.1), McCEH2 (XP_047426224.1), McCEH3 (XP_047426225.1), and McCEH4 (XP_047448502.1). They have 65.22%, 64.49%, 62.50%, and 56.96% identity, respectively, with the first 560 residues of HCEH (Fig. 7) whose three-dimensional structure is known (Moore et al., 2001). Unlike mammals, which have only one gene encoding CEH, most fish have between two and five CEH genes (Tang et al., 2022). Fish CEHs have a shorter sequence than HCEH and do not have a C-terminal end consisting of proline-rich repeat sequences as in mammals. Four CEHs have also been found in an herbivorous fish species, the monkeyface prickleback (Cebidichthys violaceus) (Heras et al., 2020). In this fish, it has been proposed that CEHs are involved in galactolipid digestion (Heras et al., 2020; Sahaka et al., 2020), by analogy with HCEH, which has galactolipase activity (Amara et al., 2009).
Since M. cephalus does not have PLRP2, it appears that its four McCEHs are good candidates for the digestion of galactolipids, the main membrane lipids in photosynthetic organisms such as microalgae and diatoms. Moreover, the identification of these enzymes is consistent with the partial biochemical characterization of the only lipase from yellow mullet studied to date (Aryee et al., 2007). To support this hypothesis, we built three-dimensional molecular models of each of these McCEHs. Their superposition with the known structure of HCEH (PDB: 1JMY; Moore et al., 2001) illustrates their very high sequence homology, which translates to the tertiary level (Fig. 8A). All residues involved in the catalytic triad are conserved, including the catalytic serine located and accessible at the bottom of a solvent-accessible cavity. To determine if this cavity could accommodate a galactolipid molecule, we first modeled a digalactosyl dilinoleoyl glycerol (DGDG) molecule into the active site of HCEH and determined its most probable binding site by molecular docking (Fig. 8B). We then looked at whether this localization and conformation of DGDG could fit into the active site cavities of M. cephalus CEHs. The best match was found with McCEH3 (Fig. 8C), where it is almost unnecessary to modify the conformation of DGDG. For the other three CEHs, adjustments are necessary, especially in McCEH4, where a loop of the polypeptide chain enters the active site and creates steric hindrance (cyan-colored model in Fig. 8C).
 |
Fig 7 Alignment of the protein sequences of pancreatic carboxylesterases from Mugil cephalus with that of human pancreatic HCEH. The secondary structure elements of the latter, identified in the known 3D structure (Moore et al., 2001), are indicated above the sequence. Only the sequence of the first 560 amino acids is shown here. The C-terminal end up to residue 753, consisting of proline-rich repeat sequences, is truncated. The signal peptide cleavage site is indicated by a vertical arrow. The three amino acids of the catalytic triad (Ser-Asp-His) are indicated by asterisks. This sequence alignment was obtained and depicted using the Clustal Omega (Sievers et al., 2011) and Espript (Gouet, et al., 2003) programs. |
 |
Fig. 8 Three-dimensional molecular models of pancreatic carboxylesterases from Mugil cephalus. (A) 3D models, presented in "ribbon" form, of McCEH1 (green), McCEH2 (yellow), McCEH3 (magenta), and McCEH4 (cyan), superimposed onto the known 3D structure of human pancreatic HCEH (grey; (Moore et al., 2001)). The atoms of the catalytic serine are shown as red spheres. (B) Structure of HCEH showing molecular surfaces (grey) and a DGDG molecule (green stick model with oxygen atoms in red) positioned in the active site by molecular docking. (C) 3D model of McCEH3 showing molecular surfaces (magenta) and the same DGDG molecule, which also fits well into the active site of McCEH3. These images were generated using the PyMol program (Schrodinger, 2010). |
4 Conclusion
The fatty acid composition of yellow mullet oil reflects the diet of this fish, which is largely composed of diatoms. These diatoms are rich in EPA (C20:5 n-3), which is the most abundant fatty acid in fish oil. The presence of 16:4n-1 as a trophic marker also support the diatom origin of fatty acids found in fish oil. Our work thus illustrates the transfer of fatty acids within the food chain. For this to occur, the yellow mullet must be able to digest the lipids from diatoms, absorb the released fatty acids, and use them for the resynthesis of its own acylglycerolipids, including triacylglycerols. Genome analysis of M. cephalus has allowed us to identify four carboxylic ester hydrolases homologous to human pancreatic carboxylic ester hydrolase or bile salt-stimulated lipase, which could therefore possess galactolipase activity and contribute to the digestion of galactolipids from the photosynthetic membranes of diatoms. Futher characterization of these lipases is in progress.
These results provide a better characterization of the yellow mullet fish oil produced by the Imrâguens (Fig. 3) and known for its beneficial health effects. We confirm its richness in polyunsaturated omega-3 fatty acids. The Imrâguens promote the fishing of yellow mullet by commercializing this oil, as well as dried fish fillets and bottarga. Various parts of the fish are thus already valorized to a large extent. However, our results suggest an additional valorization of yellow mullet by-products with the exploitation of viscera. These could prove to be an interesting source of enzymatic activities, particularly lipolytic enzymes. We plan to characterize the lipases present in these viscera and evaluate the possibility of producing enzymatic extracts from what is currently considered waste.
Supplementary Material
Fragments of 3-pyridylcarbinol derivative of 6,9,12,15-hexadecatetraenoic acid (16:4n- 1) identified by mass spectrometry
L’huile de mulet jaune des Imrâguens du Banc d’Arguin en Mauritanie : Un exemple de transfert des acides gras polyinsaturés des diatomées vers le poisson, au sein de la chaîne alimentaire.
Access hereAcknowledgments
We would like to thank the Scientific Council of the Banc d’Arguin National Park for their support of our project and the management of PNBA for their hospitality at the Mamghar and Iwik base camps. We extend our gratitude to Madame Salembouha Mohamed and her family (village of Mamghar) for their warm welcome and for demonstrating the steps involved in the production of yellow mullet fish oil and bottarga. We also acknowledge financial support from CNRS through the Visiting Fellowships program as part of the CNRS-Africa 2023 initiative. We acknowledge the support of the Heliobiotech platform from the BIAM laboratory (Dr. Yonghua Li-Beisson), Cadarache, France, for GC-MS and UPLC-MS analysis.
Conflicts of interest
The authors declare no existing conflict of interest.
Author contribution statement
Sidi Boune M.V.: Investigation and formal analysis, Funding Acquisition, Writing − Review & Editing; Sidi Cheikh M.A.: Resources; Ba M.A.: Resources; Barouh N.: Investigation and formal analysis; Legeret B.: Investigation, formal analysis and writing; Ould Souvi S.M.: Writing − Review & Editing; Deida M.V.: Funding Acquisition, Resources; Launay H.: Writing − Review & Editing; Funding Acquisition; Carrière F.: Formal analysis, Methodology; Writing − Original Draft Preparation.
References
- Abida H, Dolch JL, Mei C, Villanova V, Conte M, Block AM, Finazzi G, Bastien O, Tirichine L, Bowler C, Rebeille F, Petroutsos D, Jouhet J, Marechal E. 2015. Membrane glycerolipid remodeling triggered by nitrogen and phosphorus starvation in Phaeodactylum tricornutum. Plant Physiol 167: 118–136. [CrossRef] [PubMed] [Google Scholar]
- Alonso DL, Belarbi HE, Fernandez-Sevilla JM, Rodriguez-Ruiz J, Molina Grima E. 2000. Acyl lipid composition variation related to culture age and nitrogen concentration in continuous culture of the microalga Phaeodactylum tricornutum. Phytochemistry 54: 461–471. [CrossRef] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers WE, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol, 215: 403–10. [CrossRef] [PubMed] [Google Scholar]
- Amara S, Barouh N, Lecomte J, Lafont D, Robert S, Villeneuve P, De Caro A, Carriere F. 2010. Lipolysis of natural long chain and synthetic medium chain galactolipids by pancreatic lipase-related protein 2. Biochim Biophys Acta 1801: 508–16. [CrossRef] [PubMed] [Google Scholar]
- Amara S, Lafont D, Fiorentino B, Boullanger P, Carriere F, De Caro A. 2009. Continuous measurement of galactolipid hydrolysis by pancreatic lipolytic enzymes using the pH-stat technique and a medium chain monogalactosyl diglyceride as substrate. Biochim Biophys Acta 1791: 983–90. [CrossRef] [PubMed] [Google Scholar]
- Andersson L, Bratt C, Arnoldsson CK, Herslof B, Olsson UN, Sternby B, Nilsson A. 1995. Hydrolysis of galactolipids by human pancreatic lipolytic enzymes and duodenal contents. J Lipid Res 36: 1392–400. [CrossRef] [PubMed] [Google Scholar]
- Aryee ANA, Simpson KB, Villalonga R. 2007. Lipase fraction from the viscera of grey mullet (Mugil cephalus). Isolation, partial purification, and some biochemical characteristics. Enzyme Microb Technol 40: 394–402. [CrossRef] [Google Scholar]
- Bedhhi MLOA, El Cafsi M, Marzouk B,Zarrouk K, Romdhane MS. 2004. Etude comparative des lipides de la boutargue du mulet à grosse tête (linné 1758) de l’océan Atlantique : Nouakchott (Mauritanie) et de la mer Méditerranée : Tunis (Tunisie). Bull l’Institut Natl Sci Tech Salammbô 31: 69–74. [Google Scholar]
- Ben Bacha A, Karray A, Daoud L, Bouchaala E, M.Bou Ali, Gargouri Y, Ben Ali Y. 2011. Biochemical properties of pancreatic colipase from the common stingray Dasyatis pastinaca. Lipids Health Dis 10: 69. [CrossRef] [PubMed] [Google Scholar]
- Bernardon M, Vall MOM. 2004. Le Mulet en Mauritanie : biologie, écologie, pêche et aménagement. In Programme Régional de Conservation de la zone côtière et Marine en Afrique de l’ouest (PRCM), edited by Fondation internationale du Banc d’Arguin (FIBA), 53. Nouakchott, Mauritania:IUCN, FIBA, PRCM), Wetlands International, WWF International. [Google Scholar]
- Bochert R, Bernolle D, Grunow B. 2023. Comparison of fatty acid composition of the eggs of wild and farmed Coregonus maraena and the influence of feed. Fish Aquat Life 31: 1–14. [CrossRef] [Google Scholar]
- Bodmer MW, Angal S, Yarranton TG, Harris JT, Lyons A, King JD, Pieroni G, Riviere C, Verger R, Lowe PA. 1987. Molecular cloning of a human gastric lipase and expression of the enzyme in yeast. Biochim Biophys Acta 909: 237–244. [CrossRef] [PubMed] [Google Scholar]
- Boulay S. 2010. Statut d’exception du Mulet jaune dans la société maure (Mauritanie) : gibier des pêcheurs imrâgen, viande des pasteurs nomade. Anthropozoologica 45: 101–114. [CrossRef] [Google Scholar]
- Carrière F, Withers-Martinez C, van Tilbeurgh H, Roussel A, Cambillau C, Verger R. 1998. Structural basis for the substrate selectivity of pancreatic lipases and some related proteins. Biochim Biophys Acta 1376: 417–432. [CrossRef] [PubMed] [Google Scholar]
- Cartens M, Grima ME, Medina RA, Giménez AG, González JI. 1996. Eicosapentaenoic acid (20: 5n-3) from the marine microalga Phaeodactylum tricornutum. J Am Oil Chem Soc 73: 1025–1031 [CrossRef] [Google Scholar]
- Crenon I, Jayne S, Kerfelec B, Hermoso J, Pignol D, Chapus C. 1998. Pancreatic lipase-related protein type 1: a double mutation restores a significant lipase activity. Biochem Biophys Res Commun 246: 513–517. [CrossRef] [PubMed] [Google Scholar]
- De Cenival P, Monod T. 1938. Description de la côte d’Afrique de Ceuta au Sénégal par Valentin Fernandes (1506-1507) (Larose: Paris). [Google Scholar]
- Ewan Trégarot E, Catry T, Pottier A, Cornet C, Maréchal JP, Fayad V,Sidi Cheick MA, David G, Dia DA, Fall DA, Sarr O, El Valy Y, Wagne HO, Meisse B,Kane AE, Ball CA, Haidallah SM, Braham BC, Dia M, Hamid AL M, Rey-Valette H,Salles MJ, Ly D, Cissé CB, Failler P. 2019. Évaluation des services écosystémiques du Banc d’Arguin, Mauritanie : rapport final.“ In, 1–368. University of portsmouth; CEE-M. Centre d’Economie de l’Environnement − Montpellier; IRD. Institut de Recherche pour le Développement; Nova Blue Environnement; IMROP. Institut Mauritanien de Recherches Océanographiques et des Pêches; FFEM. Fonds Français pour l’Environnement Mondial; PNBA. Parc national du Banc d’Arguin; AFD. Agence Française de Développement; BaCoMab. Fonds fiduciaire du Banc d’Arguin et de la biodiversité côtière et marine. [Google Scholar]
- Gedi MA, Magee JK, Darwish R, Eakpetch P, Young I, Gray DA. 2019. Impact of the partial replacement of fish meal with a chloroplast rich fraction on the growth and selected nutrient profile of zebrafish (Danio rerio). Food Funct 10: 733–745. [CrossRef] [PubMed] [Google Scholar]
- Gerard C, Lebrun R, Lemesle E, Avilan L, Chang SK, Jin E, Carriere F, Gontero B, Launay H. 2022. Reduction in phosphoribulokinase amount and re-routing metabolism in Chlamydomonas reinhardtii CP12 mutants. Int J Mol Sci 23: 2710. [CrossRef] [PubMed] [Google Scholar]
- Gouet P, Robert X, Courcelle E. 2003. ESPript/ENDscript: extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res 31: 3320–3. [CrossRef] [PubMed] [Google Scholar]
- Harvey DJ. 1998. Picolinyl esters for the structural determination of fatty acids by GC/MS. Mol Biotechnol 10: 251–260. [CrossRef] [PubMed] [Google Scholar]
- Harvey DJ. 1984. Picolinyl derivatives for the structural determination of fatty acids by mass spectrometry: applications to polyenoic acids, hydroxy acids, di-acids and related compounds. Biol Mass Spectr 11: 340–347. [CrossRef] [Google Scholar]
- Heras J, Chakraborty M, Emerson JJ, German DP. 2020. Genomic and biochemical evidence of dietary adaptation in a marine herbivorous fish. Proc Biol Sci 287: 20192327. [PubMed] [Google Scholar]
- Kelley LA, Mezulis S, Yates MC, Wass NM, Sternberg MJ. 2015. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10: 845–858. [CrossRef] [PubMed] [Google Scholar]
- Kergomard J, Carriere F, Paboeuf G, Barouh N, Bourlieu-Lacanal C, Vie V. 2022. Modulation of gastric lipase adsorption onto mixed galactolipid-phospholipid films by addition of phytosterols. Colloids Surf B 220: 112933. [CrossRef] [Google Scholar]
- Li Y, Monroig O, Zhang L, Wang S, Zheng X, Dick RJ, You C, Tocher DR. 2010. Vertebrate fatty acyl desaturase with Delta4 activity. Proc Natl Acad Sci U S A 107: 16840–16845. [CrossRef] [PubMed] [Google Scholar]
- Linder M, Fanni J, Parmentier M. 2004. Extraction, fractionnement et concentration des huiles marines. Oléagineux, Corps Gras, Lipides 11: 123–130. [CrossRef] [EDP Sciences] [Google Scholar]
- Lombardo, D, Fauvel J, Guy O. 1980. Studies on the substrate specificity of a carboxyl ester hydrolase from human pancreatic juice. I. Action on carboxyl esters, glycerides and phospholipids. Biochim Biophys Acta 611: 136–146. [CrossRef] [PubMed] [Google Scholar]
- Lombardo D, Guy O. 1980. Studies on the substrate specificity of a carboxyl ester hydrolase from human pancreatic juice. II. Action on cholesterol esters and lipid-soluble vitamin esters. Biochim Biophys Acta 611: 147–155. [CrossRef] [PubMed] [Google Scholar]
- Mekhalfi M, Amara S, Robert S, Carriere F, Gontero B. 2014. Effect of environmental conditions on various enzyme activities and triacylglycerol contents in cultures of the freshwater diatom, Asterionella formosa (Bacillariophyceae). Biochimie 101: 21–30. [CrossRef] [PubMed] [Google Scholar]
- Michaelis H. 1993. Food items of the grey mullet Mugil cephalus in the Banc d’Arguin area (Mauritania). Hydrobiologia 258: 175–83. [CrossRef] [Google Scholar]
- Monod T. 2001. Maxence au désert. Un voyage en Mauritanie (Actes Sud: Arles). [Google Scholar]
- Moore SA, Kingston LR, Loomes MK, Hernell O, Blackberg L, Baker MH, Baker EN. 2001. The structure of truncated recombinant human bile salt-stimulated lipase reveals bile salt-independent conformational flexibility at the active-site loop and provides insights into heparin binding. J Mol Biol 312: 511–523. [CrossRef] [PubMed] [Google Scholar]
- Moreau H, Gargouri Y, Lecat D, Junien J-L., Verger R. 1988. Screening of preduodenal lipases in several mammals. Biochim Biophys Acta 959: 247–252. [CrossRef] [PubMed] [Google Scholar]
- Noray-Dardenne ML. 2006. Le Livre des Imraguen (Buchet-Chastel: Paris). [Google Scholar]
- Oboh A, Kabeya N, Carmona-Antonanzas G, Castro LCF, Dick RJ, Tocher RD, Monroig O. 2017. Two alternative pathways for docosahexaenoic acid (DHA, 22: 6n-3) biosynthesis are widespread among teleost fish. Sci Rep 7: 3889. [CrossRef] [PubMed] [Google Scholar]
- Oku H, Koizumi N, Okumura T, Kobayashi T, Umino T. 2006. Molecular characterization of lipoprotein lipase, hepatic lipase and pancreatic lipase genes: effects of fasting and refeeding on their gene expression in red sea bream Pagrus major. Comp Biochem Physiol B Biochem Mol Biol 145: 168–178. [CrossRef] [PubMed] [Google Scholar]
- Parma L, Bonaldo A, Pirini M, Viroli C, Parmeggiani A, Bonvini E, Gatta PP. 2015. Fatty acid composition of eggs and its relationships to egg and larval viability from domesticated common sole (Solea solea) breeders. Reprod Domest Anim 50: 186–194. [CrossRef] [PubMed] [Google Scholar]
- Qiao H, Conga C, Suna C, Li B, Wang J, Zhang L. 2016. Effect of culture conditions on growth, fatty acid composition and DHA/EPA ratio of Phaeodactylum tricornutum. Aquaculture 452: 311–317. [CrossRef] [Google Scholar]
- Rosa A, Piras A, Nieddu M, Putzu D, Cesare Marincola F,Falchi AM. 2016. Mugil cephalus roe oil obtained by supercritical fluid extraction affects the lipid profile and viability in cancer HeLa and B16F10 cells. Food Funct 7: 4092–4103. [CrossRef] [PubMed] [Google Scholar]
- Roussel A, de Caro J, Bezzine S, Gastinel L, de Caro A, Carriere F, Leydier S, Verger R, Cambillau C. 1998. Reactivation of the totally inactive pancreatic lipase RP1 by structure- predicted point mutations. Proteins − Struct Funct Genet 32: 523–531. [CrossRef] [Google Scholar]
- Rudd EA, Brockman HL. 1984. Pancreatic carboxyl ester lipase (cholesterol esterase).’ in Borgström B. and Brockman H.L. (eds.), Lipases ( Elsevier Science Publishers: Amsterdam). [Google Scholar]
- Saele O, Nordgreen A, Olsvik AP, Hamre K. 2010. Characterization and expression of digestive neutral lipases during ontogeny of Atlantic cod (Gadus morhua). Comp Biochem Physiol A Mol Integr Physiol 157: 252–259. [CrossRef] [PubMed] [Google Scholar]
- Sahaka M, Amara S, Wattanakul J, Gedi AM, Aldai N, Parsiegla G, Lecomte J, Christeller TJ, Gray D, Gontero B, Villeneuve P, Carriere F. 2020. The digestion of galactolipids and its ubiquitous function in Nature for the uptake of the essential alpha-linolenic acid. Food Funct 11: 6710–67144. [CrossRef] [PubMed] [Google Scholar]
- Sargent JR. 1995. Origins and functions of egg lipids: nutritional implications in N.R. Bromage and R.J. Roberts (eds.), Broodstock Management and Egg and Larval Quality. Oxford: Blackwell Science Ltd. [Google Scholar]
- Schrodinger, L.L.C. 2010. The PyMOL Molecular Graphics System. [Google Scholar]
- Seeliger D, de Groot BL. 2010. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J Comput Aided Mol Des 24: 417–422. [CrossRef] [PubMed] [Google Scholar]
- Shekhar MS, Katneni KV, Jangam KA, Krishnan K, Prabhudas KS, Jani Angel JR, Sukumaran K, Kailasam M, Jena J. 2022. First report of chromosome-level genome assembly for Flathead Grey Mullet, Mugil cephalus (Linnaeus, 1758). Front Genet 13: 911446. [CrossRef] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson JT, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson DJ, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7: 539. [CrossRef] [PubMed] [Google Scholar]
- Sternby B, Engstrom A, Hellman U. 1984. Purification and characterization of pancreatic colipase from the dogfish (Squalus acanthius). Biochim Biophys Acta 789: 159–163. [CrossRef] [PubMed] [Google Scholar]
- Tang SL, Liang FX, He S, Li L, Alam SM, Wu J. 2022. Comparative study of the molecular characterization, evolution, structure modeling of digestive lipase genes reveals the different evolutionary selection between mammals and fishes. Front Genet 13: 909091. [CrossRef] [PubMed] [Google Scholar]
- Thomson JM. 1990. Mugilidae. In Check-list of the fishes of the eastern tropical Atlantic (CLOFETA), edited by J. C. Quero, J. C. Hureau, Karrer C, A. Post and Saldanha L. Lisbon, Paris: JNICT, SEI and UNESCO, pp. 855-859. [Google Scholar]
- Trott O, Olson AJ. 2010. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31: 455–461. [CrossRef] [PubMed] [Google Scholar]
- Wattanakul J, Sahaka M, Amara S, Mansor S, Gontero B, Carriere F, Gray D. 2019. In vitro digestion of galactolipids from chloroplast-rich fraction (CRF) of postharvest, pea vine field residue (haulm) and spinach leaves. Food Funct 10: 7806–7817. [CrossRef] [PubMed] [Google Scholar]
- Whitfield AK, Panfili J, Durand JD. 2012. A global review of the cosmopolitan flathead mullet Mugil cephalus Linnaeus 1758 (Teleostei: Mugilidae), with emphasis on the biology, genetics, ecology and fisheries aspects of this apparent species complex. Rev Fish Biol Fisheries 22: 641–681. [CrossRef] [Google Scholar]
- Wiegand MD. 1996. Composition, accumulation and utilization of yolk lipids in teleost fish. Rev Fish Biol Fisheries 6: 259–286. [CrossRef] [Google Scholar]
- Youson JH, Al-Mahrouki AA, Amemiya Y, Graham CL, Montpetit JC, Irwin DM. 2006. The fish endocrine pancreas: review, new data, and future research directions in ontogeny and phylogeny. Gen Comp Endocrinol 148: 105–115. [CrossRef] [PubMed] [Google Scholar]
- Zhao N, Guo BH, Jia L, Dong Z, Zhang B. 2022. The genome assembly of flathead grey mullet Mugil cephalus. Front Marine Sci 9: 988397. [CrossRef] [Google Scholar]
Cite this article as: Sidi Boune MV, Cheikh MAS, Ba MA, Barouh N, Legeret B, Souvi SMO, Deida MV, Launay H, Carrière F. 2024. The yellow mullet fish oil from the Banc d’Arguin Imrâguens in Mauritania: an example of polyunsaturated fatty acids transfer from diatoms to the fish within the alimentary chain. OCL 31: 23. https://doi.org/10.1051/ocl/2024023
All Tables
Fatty acid composition of Yellow Mullet fish oil, bottarga (from the same fish), and lipids of the model marine diatom, Phaeodactylum tricornutum.
All Figures
 |
Fig. 1 Location of the Banc d’Arguin National Park in Mauritania. The left view is a Sentinel 2 map of the coastal marine biocenoses and terrestrial environments of the PNBA. Adapted from (Ewan Trégarot et al., 2019). |
| In the text | |
 |
Fig. 2 Preparation of bottarga. The double pouch of yellow mullet eggs is manually removed when the fish is cut. It is then tied with a string, rinsed, salted for one hour, rinsed again, and left to dry in a ventilated area. |
| In the text | |
 |
Fig. 3 Extraction of fish oil from yellow mullet heads. The cut heads are boiled in sea water in a large pot to separate the oil from the tissues, cartilage, and bones. After cooling, the oil floating on the surface is collected using a Cymbium shell and bottled. |
| In the text | |
 |
Fig. 4 The yellow mullet (Mugil cephalus) and its diet. (A) Photo of a yellow mullet (courtesy of J.D. Durand, Photographer). (B) Microscopic view of the stomach contents of the yellow mullet showing quartz grains, diatoms, and various aggregates. Reproduced with permission from (Michaelis, 1993). |
| In the text | |
 |
Fig. 5 Comparison of fatty acids from yellow mullet fish oil and total lipids from the microalga Chlamydomonas reinhardtii. Panel A and B: FAME separation by GC-MS using a polar Zebron 7HG-G007-11 capillary column. 16:4 from yellow mullet fish oil and Chlamydomonas reinhardtii show different retention times. Panel C and D: MS analysis on Thermo Orbitrap QExactive of 3-pyridinyl carbinol derivatives from yellow mullet fish oil and Chlamydomonas reinhardtii 16:4 after their separation by UPLC. The FAMEs from Chlamydomonas reinhardtii were produced from the total lipid extract of a culture performed as previously described (Gerard et al., 2022). |
| In the text | |
 |
Fig. 6 Mass spectrometry analysis on Orbitrap Q Exactive of 3-pyridiylcarbinol derivative from 16:4 n-1 isolated from yellow mullet fish oil. (A) Principle of fragmentation based on random proton abstraction on the hydrocarbon chain of 3-pyridylcarbinol fatty acid derivatives according to (Harvey, 1984). (B) Mass spectrum of 3 pyridylcarbinol 6,9,12,15-hexadecatetraenotate showing the various fragments obtained upon electronic impact. |
| In the text | |
 |
Fig 7 Alignment of the protein sequences of pancreatic carboxylesterases from Mugil cephalus with that of human pancreatic HCEH. The secondary structure elements of the latter, identified in the known 3D structure (Moore et al., 2001), are indicated above the sequence. Only the sequence of the first 560 amino acids is shown here. The C-terminal end up to residue 753, consisting of proline-rich repeat sequences, is truncated. The signal peptide cleavage site is indicated by a vertical arrow. The three amino acids of the catalytic triad (Ser-Asp-His) are indicated by asterisks. This sequence alignment was obtained and depicted using the Clustal Omega (Sievers et al., 2011) and Espript (Gouet, et al., 2003) programs. |
| In the text | |
 |
Fig. 8 Three-dimensional molecular models of pancreatic carboxylesterases from Mugil cephalus. (A) 3D models, presented in "ribbon" form, of McCEH1 (green), McCEH2 (yellow), McCEH3 (magenta), and McCEH4 (cyan), superimposed onto the known 3D structure of human pancreatic HCEH (grey; (Moore et al., 2001)). The atoms of the catalytic serine are shown as red spheres. (B) Structure of HCEH showing molecular surfaces (grey) and a DGDG molecule (green stick model with oxygen atoms in red) positioned in the active site by molecular docking. (C) 3D model of McCEH3 showing molecular surfaces (magenta) and the same DGDG molecule, which also fits well into the active site of McCEH3. These images were generated using the PyMol program (Schrodinger, 2010). |
| In the text | |
Les statistiques affichées correspondent au cumul d'une part des vues des résumés de l'article et d'autre part des vues et téléchargements de l'article plein-texte (PDF, Full-HTML, ePub... selon les formats disponibles) sur la platefome Vision4Press.
Les statistiques sont disponibles avec un délai de 48 à 96 heures et sont mises à jour quotidiennement en semaine.
Le chargement des statistiques peut être long.




