| Issue |
OCL
Volume 30, 2023
|
|
|---|---|---|
| Article Number | 10 | |
| Number of page(s) | 8 | |
| Section | Nutrition - Health | |
| DOI | https://doi.org/10.1051/ocl/2023008 | |
| Published online | 08 June 2023 | |
Médaille Chevreul
F4-neuroprostanes and F2-dihomo-isoprostanes: biomarkers and bioactive oxylipins
F4-neuroprostanes et F2-dihomo-isoprostanes : biomarqueurs et oxylipines bioactives
Institut des Biomolécules Max Mousseron IBMM, Pôle Recherche Chimie Balard, UMR 5247 Université Montpellier, CNRS, ENSCM, 1919 route de Mende, F-34293 Montpellier Cedex 05, France
* Correspondence: thierry.durand@umontpellier.fr
Received:
1
May
2023
Accepted:
13
May
2023
Under condition of oxidative stress, free radical-catalyzed peroxidation of docosahexaenoic acid (DHA) and adrenic acid (AdA) generates in vivo neuroprostanes (NeuroPs) and dihomo-isoprostanes (dihomo-IsoPs), among a large number of key products participating in many pathophysiological processes. These non-enzymatic oxygenated metabolites display a wide range of biological actions (especially DHA-metabolites), and some of them are now considered as the most reliable indicators of oxidative stress in neurogenerative, neurodevelopmental or cardiovascular diseases. In this review, we will present an overview regarding neuroprostanes and dihomo-isoprostanes and discuss about their biological interests.
Résumé
Dans des conditions de stress oxydatif, la peroxydation, catalysée par les radicaux libres de l’acide docosahexaénoïque (DHA) et de l’acide adrénique (AdA) génère, parmi un grand nombre de produits clés participant à de nombreux processus physiopathologiques, des neuroprostanes (NeuroPs) et dihomo-isoprostanes (dihomo-IsoPs). Ces métabolites oxygénés non-enzymatiques présentent de nombreuses activités biologiques, et certains d’entre eux sont désormais considérés comme les biomarqueurs les plus fiables du stress oxydatif dans les maladies neurogénératives, neurodéveloppementales ou encore cardiovasculaires. Dans cette revue, seront présentés les différents travaux autour des neuroprostanes et dihomo-isoprostanes, et notamment leur intérêt biologique.
Key words: biomarkers / docosahexaenoic acid / radical peroxidation / neuroprostanes / dihomo-isoprostanes / bioactive oxylipins / total synthesis
Mots clés : biomarqueurs / activités biologiques / peroxydation lipidique / acides gras polyinsaturés / neuroprostanes / dihomo-isoprostanes
© T. Durand et al., Published by EDP Sciences, 2023
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
In 1990, Morrow et al. (1990) highlighted the formation in man of the isoprostanes (IsoPs), isomeric to prostaglandins (PGs), by a non-enzymatic mechanism involving the radical peroxidation. These compounds are biosynthetized while arachidonic acid (C20:4 n-6, AA) is linked to the phospholipids. A large number of diastereoisomers, however with major cis-configurations between the lateral chains on the cyclopentane ring, can be generated, as well as several functions on the 5-member ring (diol, enone, hydroxyketone). The metabolites are further released from the membrane by a specific phospholipase A2 (PLA2) (Stafforini et al., 2006).
Later, the same group from the Vanderbilt University described a similar mechanism for the peroxidation of docosahexaenoic acid (C22:6 n-3, DHA), one of the main polyunsaturated fatty acids (PUFAs) in the brain, leading to neuroprostanes (NeuroPs) (Roberts et al., 1998; Nourooz-Zadeh et al., 1998).
It should be known that the lipid peroxidation of PUFAs (here DHA, Fig. 1) begins with the abstraction of an atom of hydrogen by HO., in one of the 5 bis-allylic positions, either on C-5, or C-8, or C-12 or C-15 or C-18. The pentadienyl radical formed reacts with a molecule of dioxygen to form a peroxyl radical which undergoes a cyclization and the subsequent radical formed reacts with a second molecular oxygen molecule to generate a hydroperoxide. Finally, a complete reduction yields the whole family of F4-NeuroPs with several series (4, 7, 10, 11, 13, 14, 17, and 20) (Fig. 1), when the partial reduction yields the D4- and E4-NeuroPs (hydroxyketone function of the cyclic ring) (Fig. 2). Note that these metabolites are produced as racemic in vivo.
The increase of the F4-NeuroP concentration is demonstrated in several pathologies as for example the neurodegenerative and cardiovascular diseases, and they are now considered as the best markers of the lipid peroxidation. Their quantification in urine and plasma allows a precise, non-invasive and representative measure of oxidative stress (Milne et al., 2007; Mas et al., 2008; Michel et al., 2008; Vigor et al., 2014).
The quantification processes were developed thanks to the availability of synthetic compounds provided by organic chemists able to produce large amount of pure compounds (Jahn et al., 2008). Furthermore, some metabolites that became commercially available were studied and a lot of biological activities were reported over the years (Galano et al., 2017; Ahmed et al., 2020). For example, to determine whether IsoP structures possessed intrinsic biological activities, Morrow et al. (1990) have tested the 15-F2t-IsoP to study blood pressure. The metabolite was injected in a rat kidney, in the peripheral vein or directly in the kidney, and a reduction of the blood pressure as well as the rate of filtration was observed. IsoPs and other PUFA metabolites thus have biological activities which confer them a role of mediator in a context of OS (Galano et al., 2017; Ahmed et al., 2020).
In mammals, this prolific pathway can occur with different PUFAs (Fig. 3). If DHA is enriched in the grey matter and retina, and yields to neuroprostanes (NeuroPs) after peroxidation (Roberts et al., 1998; Nourooz-Zadeh et al., 1998), another PUFA, adrenic acid (AdA, C22:4 n-6) is mainly present in the myelin (white matter) and retina and produces another class of metabolite, the dihomo-Isoprostanes (dihomo-IsoPs) (Song et al., 2008; VanRollins et al., 2008; De La Torre et al., 2014, 2015; De Felice et al., 2011). In plants, the phytoprostanes (PhytoPs) were described, resulting from the radical peroxidation of α-linolenic acid (ALA, C18:3 n-3) (Imbusch and Mueller, 2000). It should be mentioned that twelve years after the discovery of the IsoPs, Fessel et al. (2002) have highlighted a new class of metabolites containing a tetrahydrofuran core and named them isofurans (IsoFs). Later, neurofurans (NeuroFs) (De La Torre et al., 2015) and dihomo-isofurans (dihomo-IsoFs) were detected (De La Torre et al., 2014).
The lovely story of some of these molecules, especially NeuroPs and dihomo-IsoPs will be presented in this review. Several outcomes in terms of syntheses, diagnosis and biological activities, gathering almost thirty years of research, from organic chemistry knowledge through a smart multi-step synthesis strategy providing many pure compounds, to our fruitful collaborations with a large number of scientists, biologists, clinicians all around the world (Galano et al., 2013, 2015).
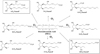 |
Fig. 1 F4-NeuroP products of DHA peroxidation. |
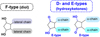 |
Fig. 2 E- and D- type products of PUFA peroxidation. |
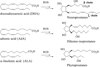 |
Fig. 3 Metabolites resulting of the radical peroxidation of PUFAs. |
2 Chemical synthesis
Since the discovery of IsoPs, our group among other developed several strategies to access PUFA metabolites with high purity and enantioselectivity (Vigor et al., 2022). Nowadays, in our group we are using the last, simple and highly stereocontrolled strategy developed in 2008 (Oger et al., 2008). This strategy is based on a bicyclic α,β-epoxy ketone intermediate (Fig. 4). To this core, and as the key steps, Horner–Wadworth–Emmons and Wittig reactions permit to plug the large diversity of lateral chains, thanks to the synthesis of the appropriate phosphonium salts or phosphonates. The allylic alcohol on the lateral chain is obtained either as (R) or (S)-epimer thanks to an enantioselective chemical reduction. These strategies have allowed us to access IsoPs, dihomo-IsoPs, NeuroPs and other PhytoPs metabolites (Fig. 4) (Brinkmann et al., 2010; Oger et al., 2010a, b, 2012; Guy et al., 2014).
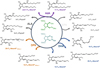 |
Fig. 4 A fully flexible approach. |
3 New biomarkers
The best technic for the analysis of such metabolites is the tandem chromatography-mass spectrometry, whether using gas or liquid chromatograph (GC-MS, LC-MS). Thanks to the development of MS2, GC-MS/MS and LC-MS/MS technics are therefore more specific and sensitive, and allow quantification of particular metabolites found in low abundance in different sample matrices. Both GC-MS/MS and LC-MS/MS have been used for quantification of a wide range of isoprostanoids. However, the derivatization process required for GC-MS analysis to improve the volatility and thermal stability of the compounds is often time-consuming and lead to low recovery yields. Indeed, this derivatization process needs to convert the carboxylic acid into pentafluorobenzyl esters (PFB) by pentafluorobenzyl bromide (PFBBr) in the presence of a catalyst such as N,N’-diisopropylethylamine, and the hydroxyl functions with either O-bis (trimethylsilyl) trifluoroacetamide + 1% trimethylchlorosilane (BSTFA+TMCS) or N-Methyl-N-trimethylsilyltrifluoroacetamide (Vigor et al., 2014) to form trimethylsilyl ether derivatives.
However, derivatization is not a mandatory for LC-MS technic. Therefore, several methods were developed for LC-MS/MS studies of PUFA metabolites (Collado-Gonzales et al., 2015; Lee et al., 2016; Sánchez-Illana et al., 2017; Rund et al., 2018). As for example, our group in collaboration with the group of Justine Bertrand-Michel was able to detect and quantify more than 50 isoprostanoids synthesized in our group, in a run a 20 min (Fig. 5) (Dupuy et al., 2016). It should be mentioned that depending of the series of isoprostanoids, this method permits to separate the two epimers of the allylic alcohol (Fig. 6). In this sense, the development of new technic and/or methods to separate diastereoisomers of PUFA metabolites is still of great interest.
 |
Fig. 5 LC-MS/MS > 50 isoprostanoids. |
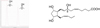 |
Fig. 6 Epimer separation for the 8(RS)-8-F3t-IsoP. |
3.1 Neuroprostanes
Our two first examples reporting the interest of F4-NeuroPs as biomarkers of pathological states were performed by Barden et al. (1996, 2004, 2012). In a recent study F4-NeuroPs, F2-IsoPs as well as IsoFs were quantified in maternal plasma and cord blood of women with pre-eclampsia and normal pregnancie. Women with pre-eclampsia had significantly elevated maternal IsoFs and F4-NeuroPs, but no F2-IsoPs. Cord blood F4-NeuroPs were elevated among neonates of mother with pre-eclampsia. Interestingly, cord blood IsoFs were approximately 5-fold higher than those found in maternal plasma. This could reflect the oxidative challenge presented at birth when there is transition from a relatively low intra-uterine oxygen environment to a significantly higher extra uterine oxygen environment. Maternal F4-NeuroPs were not significantly correlated with cord blood F4-NeuroPs in pre-eclamptic and in normal pregnancies, suggesting the origin of cord F4-NeuroPs may be independent of maternal plasma. In normal pregnancy, birth weight was negatively related to maternal F2-IsoPs, IsoFs and F4-NeuroPs.
The brain is vulnerable to oxidative insult because of high oxygen requirements for its metabolism and high PUFA composition, in particular DHA. Thus, F4-NeuroPs are considered as specific markers of brain OS. Aneurysmal subarachnoid hemorrhage (aSAH) and traumatic brain injury (TBI) are associated with devastating central nervous system (CNS) injury. Two case-controlled studies have shown a significant increase in cerebrospinal fluid (CSF) of IsoFs in aSAH and TBI patients compared with their respective age- and gender-matched controls. aSAH patients also had significantly increased levels of CSF F4-NeuroPs and F2-IsoPs. Patients with TBI had significantly increased CSF F4-NeuroPs but F2-IsoPs were similar to control (Corcoran et al., 2011). These data confirm that CNS injury, in case of aSAH or TBI, results in increased OS and as DHA is the brain major PUFA, F4-NeuroP levels in CSF could be a much more specific indicator of neurological dysfunction than F2-IsoPs. Hsieh et al. (2009) have showed that increased F4-NeuroPs in CSF of patients with aSAH correlated with poor neurological outcome. They suggested that F4-NeuroPs might be more useful than F2-IsoPs in CSF to predict outcome and interpret the role of hemorrhage in aSAH. Although Farias et al. (2008) showed increased F2-IsoPs during rat brain ischemia, the E2/D2-IsoPs were increased to a greater extent, suggesting the latter may better markers of OS in brain ischemia.
The anti-atherogenic effects of omega-3 fatty acids, eicosapentaenoic acid (EPA, C20:5 n-3) and DHA are well recognized but the impact of dietary intake on bioactive lipid mediator profiles remains unclear. Such a profiling effort may offer novel targets for future studies into the mechanism of action of omega-3 fatty acids. Gladine et al. (2014) studied the impact of DHA supplementation on the profiles of PUFA oxygenated metabolites and their contribution to atherosclerosis prevention. A special emphasis was given to the non-enzymatic metabolites knowing the high susceptibility of DHA to free radical-mediated peroxidation, and the increased OS associated with plaque formation. Atherosclerosis prone mice (LDLR2/2) received increasing doses of DHA (0, 0.1, 1 or 2% of energy) during 20 weeks leading to a dose-dependent reduction of atherosclerosis (R2 = 0.97, p = 0.02), triglyceridemia (R2 = 0.97, p = 0.01) and cholesterolemia (R2 = 0.96, p < 0.01). Targeted lipidomic analyses revealed that both the profiles of EPA and DHA and their corresponding oxygenated metabolites were substantially modulated in plasma and liver. Notably, the hepatic level of F4-NeuroPs was strongly correlated with the hepatic DHA level. Moreover, unbiased statistical analysis including correlation analyses, hierarchical cluster and projection to latent structure discriminate analysis revealed that the hepatic level of F4-NeuroPs was the variable most negatively correlated with the plaque extent (p < 0.001) and along with plasma EPA dihydroxylated metabolites was an important mathematical positive predictor of atherosclerosis prevention. Thus, oxygenated n-3 PUFA, and F4-NeuroPs, are potential biomarkers of DHA-associated atherosclerosis prevention. While these may contribute to the anti-atherogenic effects of DHA, further in vitro investigations are needed to confirm such a contention and to decipher the molecular mechanisms of action.
3.2 Dihomo-isoprostanes
Rett syndrome (RTT) is a pervasive abnormality of development affecting almost exclusively females, which is included among the autism spectrum disorders. RTT is caused in up to 95% of cases by mutations in the X-linked methyl-CpG binding protein 2 (MeCP2) genes (De Felice et al., 2012). Although over 200 different MeCp2 mutations have been reported to cause RTT, nine most frequent ones (hotspot mutations) are known to comprise more than three quarters of all the reported pathogenic mutations. The disease shows a wide phenotypical heterogeneity, with at least 4 distinct major clinical presentations, i.e., typical, preserved speech, early seizure variant, and congenital variant. First, clinical evidence indicates that F2-IsoPs and F4-NeuroPs are involved in the intimate pathogenetic mechanisms of RTT (De Felice et al., 2011). Plasma levels of free F2-IsoPs are significantly higher in the early stages of RTT, as compared with the late natural progression of typical RTT. Until 2011, it was thought that the predominant central nervous system damage in RTT occurred in gray matter. However, the relative abundance in myelin of the precursor AdA and the increased level of F2-dihomo-IsoPs, strongly confirm an early and severe damage to the brain white matter as suggested by previous brain MRI evidence (De Felice et al., 2011). Thus F2-dihomo-IsoPs can be considered early markers of lipid peroxidation in RTT. F4-NeuroPs also appear to be important biomarkers in RTT. Plasma F4-NeuroPs levels correlate with disease severity in RTT and are significantly related to neurological symptoms severity, mutation type and clinical presentation. Therefore, F4-NeuroPs may also play a major role along the biochemical pathway from MeCp2 gene mutation to clinical evidence, proving that a DHA oxidation process occurs (Cortelazzo et al., 2016).
The same group, De Felice and Signorini reported the importance of neuroprostanes and dihomo-isoprostanes in differents neurological diseases (Signorini et al., 2018), KRABBE disease (Signorini et al., 2019), cerebral adrenoleukodystrophie disease (Signorini et al., 2022) and finally in sperm capacitation (Signorini et al., 2021).
Finally, Vento and collaborators reported also the importance of neuroprostanes and dihomo-isoprostanes in Alzheimer disease and newborns (Garcia-Blanco et al., 2018; Peña-Bautista et al., 2019; Cascant-Vilaplana et al., 2021).
4 New bioactive lipids
PUFA metabolites are not only biomarkers of lipid peroxidation but also mediators of oxidant injury, and especially the NeuroPs.
4.1 Neuroprostanes
It is well reported that an enriched n-3 PUFA diet confers cardioprotective effects due primarily to the two main PUFA, EPA and DHA (GISSI-Prevenzione Investigators, 1999). A large prospective study showed that the most marked effect of DHA and EPA supplementation is a reduction of sudden cardiac death in the months following a cardiac infarction. This benefit has been explained, in part, by a reduction in arrhythmias and systolic cardiac failure. The anti-arrhythmic effects of n-3 PUFA have been confirmed in animal models of cardiac infarction by ligature of the left coronary artery and reported in 2003 by Judé et al. (2003). These and other studies in single cardiac cells have shown that EPA and DHA can modulate the activity of ion channels, the transmembrane proteins responsible for the electrical activity of the heart. However, it has been suggested that oxygenated metabolites of EPA and DHA may also play a role in these actions. In this regard, it has been shown that some of the effect of DHA on rat cardiac ion channels was due to an oxygenated metabolite of DHA, the 4(RS)-4-F4t-NeuroP (Fig. 7) (Roy et al., 2015).
Roy et al. (2015) have tested different F4-NeuroPs on arrhythmias induced by isoprenaline and stimulation frequency of isolated ventricular mice cardiac cells. Among them, some F4-NeuroPs have anti-arrhythmic effects (IC50 ≈ 100 nM). The main metabolite, the 4(RS)-4-F4t-NeuroP, showed potent dose-dependent in cellulo and also in vivo in permanent myocardial infarction (PMI) mice. At the cellular level, the mechanism of action is unlikely to be due to a β-blocker effect, but the anti-arrhythmic property can instead be explained by a rycal-like effect; in particular, stabilization of the RyR2 complex with FKBP12.6 (Andersson and Marks, 2010; Roy et al., 2017).
In 2017, Bosviel et al. (2017) reported that the 4(RS)-4-F4t-NeuroP and the 14-A4t-NeuroP possess an anti-inflammatory properties mediated by PPAR receptors, using primary microglial cells.
Lacampagne et al. (2022a, b) have tested first the 4(RS)-4-F4t-NeuroP on Ventilator Induced Diaphragm Dysfunction (VIDD) and confirmed that this metabolite permits to restore diaphragm muscular activity on VIDD mice and rat. This phenomenon is once again mediated again by RYR1 receptor. Later, Lacampagne et al. (2022a, b) validated the proof of concept on adult pig of the activities of 4(RS)-4-F4t-NeuroP and another compound on VIDD with a submicromolar inhibithory concentration and three patents were published (Le Guennec et al., 2012; Lacampagne et al., 2022a, b). (WO 2015197562 A1 20151230, EP12306519.3, EP22305460.2, EP22209466.6).
In 2020, Lee et al. (2020) reported for the first time that native 4(RS)-4-F4t-NeuroP has a regulatory role in neurons for cell survival on human SH-SYSY neuroblastoma cells.
Geng et al. (2022) have also reported a neuroprotective effect of 4(RS)-4-F4t-NeuroP on primary mouse microglial and BV2 cells and that the 4(RS)-4-F4t-NeuroP attenuate LPS-induced mitochondrial membrane potential loss in BV2 cells.
Finally, Moretti et al. (2023) reported this year that 4(RS)-4-F4t-NeuroP possess an effect on human sperm and precisely a role on sperm capacitation mediated again by a ryanodine receptor.
It should be mentioned that to date, there is no biological activity reported for the dihomo-IsoPs.
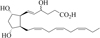 |
Fig. 7 4(RS)-4-F4t-NeuroP. |
5 Conclusion
Our understanding of the role of PUFA peroxidation in the pathogenesis of various human diseases is at an early stage. Regarding DHA, we know that free radical-induced autoxidation of this PUFA occurs in numerous pathological conditions from cardiovascular disorders to cancers and neurodegenerative diseases. Through our knowledge in organic chemistry, we can contribute to clinical and basic research by developing novel synthetic approaches and providing samples for biological and analytical studies. Several new approaches for chiral synthesis of NeuroPs and others isoprostanoids such as dihomo-IsoPs are now available. Some of these products may be used as markers for the diagnosis and management of patients and will need to be measured accurately and precisely. The contribution of each of these unique NeuroPs to tissue and organ damage has to be clearly ascertained within a complex network of signalling molecules and mediators. Despite all the work performed in this field, there is still a lot to discover, and collaborations between chemists and biologists are in this sense highly important.
Authors contribution
Camille Oger: Writting-original draft, preparation, validation. Valerie Bultel-Poncé: Methodology. Alexandre Guy: Methodology. Valérie Gros: Methodology. Guillaume Reversat: Methodology. Claire Vigor: Validation. Jean-Marie Galano: Validation. Thierry Durand: Supervision, writing–original draft, preparation, validation.
Acknowledgements
We gratefully thank all our collaborators for valorizing so well our neuroprostanes and dihomo-isoprostanes.
References
- Ahmed OS, Galano J-M, Pavlickova T, et al. 2020. Moving forward with isoprostanes, neuroprostanes and phytoprostanes – Where are we know? Essays Biochem 64: 463–484. [CrossRef] [PubMed] [Google Scholar]
- Andersson DC, Marks AR. 2010. Fixing ryanodine receptor Ca2+ leak – A novel therapeutic strategy for contractile failure in heart and skeletal muscle. Drug Discov Today: Dis Mech 7: e151–e157. [CrossRef] [Google Scholar]
- Barden A, Beilin LJ, Ritchie J, Croft KD, Walters BN, Michael CA. 1996. Plasma and urinary 8 isoprostane as an indicator of lipid peroxidation in preeclampsia and normal pregnancy. Clin Sci (Lond) 91: 711–718. [CrossRef] [PubMed] [Google Scholar]
- Barden AE, Mori TA, Dunstan JA, et al. 2004. Fish oil supplementation in pregnancy lowers F2-isoprostanes in neonates at high risk of atopy. Free Radic Res 38: 233–239. [CrossRef] [PubMed] [Google Scholar]
- Barden AE, Corcoran TB, Mas E, et al. 2012. Are isofurans and neuroprostanes increased after aneurysmal subarachnoid hemorrhage and traumatic brain injury? Antioxid Redox Signal 16: 165–169. [CrossRef] [PubMed] [Google Scholar]
- Bosviel R, Joumard-Cubizolles L, Chinetti-Gbaguidi G, et al. 2017. DHA-derived oxylipins, neuroprostanes and protectins, differentially and dose-dependently modulate the inflammatory response in human macrophages: Putative mechanisms through PPAR activation. Free Radic Biol Med 103: 146–154. [CrossRef] [PubMed] [Google Scholar]
- Brinkmann Y, Oger C, Guy A, Durand T, Galano JM. 2010. Total synthesis of 15-D2t- and 15-E2t-isoprostanes. J Org Chem 75: 2411–2414. [CrossRef] [PubMed] [Google Scholar]
- Cascant-Vilaplana MM, Sánchez-Illana Á, Piñeiro-Ramos JD, et al. 2021. Do levels of lipid peroxidation biomarkers reflect the degree of brain injury in newborns? Antiox Redox Signal 37: 1467–1475. [CrossRef] [PubMed] [Google Scholar]
- Collado-Gonzales J, Medina S, Durand T, et al. 2015. New UPLC-QqQ-MS/MS method for quantitative and qualitative determination of 10 phytoprostanes in foodstuffs in commercial olive and sunflower oils. Food Chem 178: 212–220. [CrossRef] [PubMed] [Google Scholar]
- Corcoran TB, Mas E, Barden AE, et al. 2011. Are isofurans and neuroprostanes increased after aneurysmal subarachnoid hemorrhage and traumatic brain injury? Antioxid Redox Signal 15: 2663–2667. [CrossRef] [PubMed] [Google Scholar]
- Cortelazzo A, De Felice C, Guerranti R, et al. 2016. Abnormal N-glycosylation pattern of brain nucleotide pyrophosphatase-5 (NPP-5) in Mecp2-mutant murine models of Rett syndrome. Neurosc Res 105: 28–34. [CrossRef] [Google Scholar]
- De Felice C, Signorini C, Durand T, et al. 2011. Dihomo-isoprostanes as potential early biomarkers of lipid oxidative damage in Rett syndrome 2011. J Lipid Res 52: 2287. [CrossRef] [PubMed] [Google Scholar]
- De Felice C, Signorini C, Leoncini S, et al. 2012. The role of oxidative stress in Rett syndrome: An overview. Ann NY Acad Sci 1259: 121–135. [CrossRef] [Google Scholar]
- De La Torre A, Lee YY, Oger C, et al. 2014. Synthesis, discovery and quantitation of dihomo-isofurans: Novel biomarkers of in vivo adrenic acid peroxidation. Angew Chem Int Ed 53: 6249–6252. [CrossRef] [Google Scholar]
- De La Torre A, Lee YY, Mazzoni A, et al. 2015. Total syntheses and in vivo quantitation of novel neurofuran and dihomo-isofuran derived from docosahexaenoic acid and adrenic acid. Chem Eur J 21: 2442–2446. [CrossRef] [Google Scholar]
- Dupuy A, Le Faouder P, Vigor C, et al. 2016. Simultaneous quantitative profiling of 20 isoprostanoids from omega-3 and omega-6 polyunsaturated fatty acids by LC-MS/MS in various biological samples. Anal Chim Acta 921: 46–58. [CrossRef] [PubMed] [Google Scholar]
- Farias SE, Basselin M, Chang L, Heidenreich KA, Rapoport SI, Murphy RC. 2008. Formation of eicosanoids, E2/D2 isoprostanes, and docosanoids following decapitation-induced ischemia, measured in high-energy-microwaved rat brain. J Lipid Res 49: 1990–2000. [CrossRef] [PubMed] [Google Scholar]
- Fessel JP, Porter NA, Moore KP, Sheller JR, Roberts-II LJ. 2002. Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc Natl Acad Sci USA 99: 16713–16718. [CrossRef] [PubMed] [Google Scholar]
- Galano J-M, Mas E, Barden A, et al. 2013. Isoprostanes and neuroprostanes: Total synthesis, biological activity and biomarkers of oxidative stress in humans. Prostagl Other Lipid Med 107: 95–102. [CrossRef] [Google Scholar]
- Galano J-M, Lee JC-Y, Gladine C, et al. 2015. Non-enzymatic cyclic oxygenated metabolites of adrenic, docosahexaenoic, eicosapentaenoic, a-linolenic acids; bioactivities and potential use a biomarkers. Biochim Biophys Acta 1851: 446–455. [CrossRef] [PubMed] [Google Scholar]
- Galano J-M, Lee YY, Oger C, et al. 2017. Isoprostanes, neuroprostanes, phytoprostanes. An overview of 25 years of research in chemistry and biology. Prog Lipid Res 68: 83–108. [CrossRef] [PubMed] [Google Scholar]
- Garcia-Blanco A, Pena-Bautista C, Oger C, et al. 2018. Reliable analytical method to determine new lipid peroxidation biomarkers in urine samples: Application to mild cognitive impairment due to Alzheimer disease. Talanta 184: 193–201. [CrossRef] [PubMed] [Google Scholar]
- Geng X, Galano J-M, Oger C, Sun GY, Durand T, Lee JC. 2022. Neuroprotective effects of DHA-derived peroxidation product 4(RS)-4-F4t-neuroprostane on microglia. Free Radic Biol Med 185: 1–5. [CrossRef] [PubMed] [Google Scholar]
- GISSI-Prevenzione Investigators. 1999. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet 354: 447. [CrossRef] [PubMed] [Google Scholar]
- Gladine C, Newman JW, Durand T, et al. 2014. Lipid profiling following intake of omega 3 fatty acid DHA identifies the peroxidized metabolites DHA F4-neuroprostanes as the best predictors of atherosclerosis prevention. PLoS ONE 9: e89393. [CrossRef] [PubMed] [Google Scholar]
- Guy A, Oger C, Heppekausen J, et al. 2014. Oxygenated metabolites of n-3 polyunsaturated fatty acid as potential oxidative stress biomarkers: Total synthesis of 8-F3t-IsoP, 10-F4t-NeuroP, and [D4]-10-F4t-NeuroP. Chem Eur J 20: 6374–6380. [CrossRef] [PubMed] [Google Scholar]
- Hsieh YP, Lin CL, Shiue AL, et al. 2009. Correlation of F4-neuroprostanes levels in cerebrospinal fluid with outcome of aneurysmal subarachnoid hemorrhage in humans. Free Radic Biol Med 47: 814–824. [CrossRef] [PubMed] [Google Scholar]
- Imbusch R, Mueller MJ. 2000. Formation of isoprostane F2-like compounds (phytoprostanes F1) from α-linolenic acid in plants. Free Radical Biol Med 28: 720–726. [CrossRef] [Google Scholar]
- Jahn U, Galano JM, Durand T. 2008. Beyond prostaglandins chemistry and biology of cyclic oxygenated metabolites formed by free-radical pathways from polyunsaturated fatty acids. Angew Chem Int Ed 47: 5894–5951. [CrossRef] [Google Scholar]
- Judé S, Bedut S, Roger S, et al. 2003. Peroxidation of docosahexaenoic acid is responsible for its effects on ITO and ISS in rat ventricular myocytes. Br J Pharmacol 139: 816. [CrossRef] [PubMed] [Google Scholar]
- Lacampagne A, Galano J-M, Oger C, et al. Eur Patent (05 April 2022, EP22305460. 2). [Google Scholar]
- Lacampagne A, Galano J-M, Oger C, et al. Eur Patent (22 November 2022, EP22209466. 6). [Google Scholar]
- Lee YY, Galano J-M, Oger C, et al. 2016. Lipids 51: 1217–1229. [CrossRef] [PubMed] [Google Scholar]
- Lee YY, Galano J-M, Leung HH, et al. 2020. Non-enzymatic oxygenated metabolite of docosahexaenoic acid, 4(RS)-4-F4t-neuroprostane, act as bioactive lipid molecule in neuronal cell 2020. FEBS Lett 594: 1797–1808. [CrossRef] [PubMed] [Google Scholar]
- Le Guennec J-Y, Galano JM, Oger C, et al. Eur Patent (5 December 2012, EP12306519. 3). [Google Scholar]
- Mas E, Michel F, Guy A, et al. 2008. Quantification of urinary F2-isoprostanes with 4(RS)-F4t-neuroprostane as an internal standard using gas-chromatography mass spectrometry: Application to polytraumatized patients. J Chromatrogr B 10: 5087–5090. [Google Scholar]
- Michel F, Bonnefont-Rousselot D, Mas E, Drai J, Thérond P. 2008. Biomarkers of lipid peroxidation: Analytical aspects. Ann Biol Clin 66: 605. [PubMed] [Google Scholar]
- Milne GL, Yin H, Brooks JD, Sanchez S, Roberts II LJ, Morrow JD. 2007. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzymol 433: 113–126. [CrossRef] [PubMed] [Google Scholar]
- Moretti E, Signorini C, Noto D, et al. 2023. F4-neuroprostanes effects on human sperm 2023. Int J Mol Sci 24: 935. [CrossRef] [PubMed] [Google Scholar]
- Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts II LJA. 1990. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci USA 87: 9383–9387. [CrossRef] [PubMed] [Google Scholar]
- Nourooz-Zadeh J, Liu EH, Anggard E, Halliwell B. 1998. F4-isoprostanes: A novel class of prostanoids formed during peroxidation of docosahexaenoic acid (DHA). Biochem Biophys Res Commun 242: 338. [CrossRef] [PubMed] [Google Scholar]
- Oger C, Brinkmann Y, Bouazzaoui S, Durand T, Galano J-M. 2008. Stereocontrolled access to isoprostanes via a bicyclo[3.3.0]octene framework. Org Lett 10: 5087–5090. [CrossRef] [PubMed] [Google Scholar]
- Oger C, Marton Z, Brinkmann Y, et al. 2010a. New insight in lipase-catalyzed regioselective monoacetylation of unsymmetrical 1,5-primary diols. J Org Chem 75: 1892–1897. [CrossRef] [PubMed] [Google Scholar]
- Oger C, Bultel-Poncé V, Guy A, et al. 2010b. The handy use of Brown’s catalyst for a skipped diyne deuteration: Application to the synthesis of a d4-labeled-F4t-neuroprostane. Chem Eur J 16: 13976–13980. [CrossRef] [Google Scholar]
- Oger C, Bultel-Poncé V, Guy A, Durand T, Galano J-M. 2012. Total synthesis of isoprostanes derived from AdA and EPA. Eur J Org Chem 2012: 2621–2634. [CrossRef] [Google Scholar]
- Peña-Bautista C, Vigor C, Galano J-M, et al. 2019. New screening approach for Alzheimer’s disease risk assessment from urine lipid peroxidation compounds. Sci Rep 9: 14244. [CrossRef] [PubMed] [Google Scholar]
- Roberts II LJ, Montine TJ, Markesbery WR, et al. 1998. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem 273: 13605–13612. [CrossRef] [PubMed] [Google Scholar]
- Roy J, Oger C, Thireau J, et al. 2015. Nonenzymatic lipid mediators neuroprostanes exert the anti-arrhythmic properties of docosahexenoic acid. Free Radic Biol Med 86: 269–278. [CrossRef] [PubMed] [Google Scholar]
- Roy J, Fauconnier J, Oger C, et al. 2017. Non-enzymatic oxidized metabolite of DHA, 4(RS)-4-F4t neuroprostane protects the heart against reperfusion injuries. Free Radic Biol Med 102: 229–239. [CrossRef] [PubMed] [Google Scholar]
- Rund KM, Ostermann AI, Kutzner L, et al. 2018. Development of an LC-(ESI-)-MS/MS method for the simultaneous quantification of 35 isoprostanes and isofurans derived from the major n3 and n6 PUFA. Anal Chim Acta 1037: 63–74. [CrossRef] [PubMed] [Google Scholar]
- Sánchez-Illana Á, Thayyil S, Montaldo P, et al. 2017. Novel free-radical mediated lipid peroxidation biomarkers in newborn plasma. Anal Chim Acta 996: 88–97. [CrossRef] [PubMed] [Google Scholar]
- Signorini C, De Felice C, Durand T, et al. 2018. Relevance of 4-F4t-neuroprostane and 10-F4t-neuroprostane to neurological diseases. Free Radic Biol Med 115: 278–287. [CrossRef] [PubMed] [Google Scholar]
- Signorini C, Cardile V, Pannuzzo G, et al. 2019. Increased isoprostanoid levels in brain from murine model of Krabbe disease – Relevance of isoprostanes, dihomo-isoprostanes and neuroprostanes to disease severity. Free Radic Biol Med 139: 46–54. [CrossRef] [PubMed] [Google Scholar]
- Signorini C, Moretti E, Noto D, et al. 2021. F4-neuroprostanes: A role in sperm capacitation. Life 11: 655. [CrossRef] [PubMed] [Google Scholar]
- Signorini C, De Felice C, Durand T, et al. 2022. Isoprostanoid plasma levels are relevant to cerebral adrenoleukodystrophy disease. Life 12: 146. [CrossRef] [PubMed] [Google Scholar]
- Stafforini DM, Sheller JR, Blackwell TS, et al. 2006. Release of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J Biol Chem 281: 4616–4623. [CrossRef] [PubMed] [Google Scholar]
- Song W-L, Lawson JA, Reilly D, et al. 2008. Neurofurans, novel indices of oxidant stress derived from docosahexaenoic acid. J Biol Chem 283: 6–16. [CrossRef] [PubMed] [Google Scholar]
- VanRollins M, Woltjer RL, Yin H, Morrow JD, Montine TJ. 2008. F2-dihomo-isoprostanes arise from free radical attack on adrenic acid. J Lipid Res 49: 995. [CrossRef] [PubMed] [Google Scholar]
- Vigor C, Bertrand-Michel J, Pinot E, et al. 2014. Non-enzymatic lipid oxidation products in biological systems: Assessment of the metabolites from polyunsaturated fatty acids. J Chromatogr B 964: 65–78. [CrossRef] [Google Scholar]
- Vigor C, Balas L, Guy A, et al. 2022. Isoprostanoids, isofuranoids and isoketals – From synthesis to lipidomics. Eur J Org Chem 22: e202101523. [Google Scholar]
Cite this article as: Durand T, Bultel-Poncé V, Guy A, Gros V, Reversat G, Vigor C, Galano J-M, Oger C. 2023. F4-neuroprostanes and F2-dihomo-isoprostanes: biomarkers and bioactive oxylipins. OCL 30: 10.
All Figures
 |
Fig. 1 F4-NeuroP products of DHA peroxidation. |
| In the text | |
 |
Fig. 2 E- and D- type products of PUFA peroxidation. |
| In the text | |
 |
Fig. 3 Metabolites resulting of the radical peroxidation of PUFAs. |
| In the text | |
 |
Fig. 4 A fully flexible approach. |
| In the text | |
 |
Fig. 5 LC-MS/MS > 50 isoprostanoids. |
| In the text | |
 |
Fig. 6 Epimer separation for the 8(RS)-8-F3t-IsoP. |
| In the text | |
 |
Fig. 7 4(RS)-4-F4t-NeuroP. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.




