| Issue |
OCL
Volume 18, Number 4, Juillet-Août 2011
Lipids and Brain II. Actes des Journées Chevreul 2011 (Première partie)
|
|
|---|---|---|
| Page(s) | 202 - 207 | |
| Section | PUFA and Depression | |
| DOI | https://doi.org/10.1051/ocl.2011.0395 | |
| Published online | 15 July 2011 | |
Spadin, a Sortilin-derived peptide: a new concept in the antidepressant drug design
Institut de pharmacologie moléculaire et cellulaire, CNRS, Université de Nice Sophia Antipolis, 660 Route des Lucioles, 06560
Valbonne, France
Depression is the most common of psychiatric illnesses. The design of effective treatments for this disorder is a challenging process. Recently, the two-pore domain potassium channel TREK-1 has been identified as a new target in depression, and its antagonists might become effective antidepressants. Deletion of TREK-1 gene results in a depression-resistant phenotype that mimics antidepressant treatments. Here, we validate the fast antidepressant effects of spadin, a secreted peptide derived from the propeptide generated by the maturation of the sortilin receptor and acting through TREK-1 inhibition.
Key words: Spadin / TREK-1 channel / depression / behavior
© John Libbey Eurotext 2011
Depression is a devastating illness that affects ∼17% of the population at some point in life, resulting in major social and economic consequences (Kessler et al., 2005). The use of antidepressants has an overall low clinical efficacy as full remission only occurs in one-third of the patients. In the case of response, side effects are often observed, as well as a delay in the onset of therapeutic efficiency. Most antidepressants increase levels of the monoamine serotonin (5-HT) and/or noradrenaline (NA), suggesting that biochemical imbalances within the 5-HT/NA systems may underlie the pathogenesis of this disorder (Nestler et al., 2002; Krishnan and Nestler, 2008). To date, the mainstay of antidepressant treatments is constituted by selective serotonin reuptake inhibitors, which inhibit the 5-HT reuptake pump. Improving the treatment of depression is challenging. Recently, mouse models of depression have highlighted the putative role of the TREK-1 channel in the mechanisms of action of antidepressants. The TREK-1 channel (figure 1A) belongs to the particular family of two-pore-domain potassium channels (Lesage and Lazdunski, 2000). It is open at membrane potentials in physiological conditions and contributes to the background or leak currents that set the resting potential and oppose depolarizing influences. TREK-1 is activated by volatile general anesthetics, membrane stretch, heat, acidosis, but also by riluzole, polyunsaturated fatty acids and lysophospholipids (Lesage and Lazdunski, 2000; Honore, 2007). These openers prevent neuronal death in animal models of epilepsy, stroke, global, retinal and spinal ischemia (Blondeau et al., 2002a,b; Blondeau et al., 2007; Ettaiche et al., 1999; Heurteaux et al., 2006a; Lang-Lazdunski et al., 1999, 2003; Lauritzen et al., 2000). The phenotyping of TREK1 deficient mice (kcnk2-/-) has identified a major role for TREK1 channels in the control of epileptogenesis, general anesthesia, polyunsaturated fatty acids-induced neuroprotection (Heurteaux et al., 2004) and pain (Alloui et al., 2006; Noel et al., 2009). The TREK-1 channel is physiologically blocked by phosphorylation processes such as phosphorylation resulting from Gs or Gq G-proteins. It is also inhibited by SSRIs such as fluoxetine (Heurteaux et al., 2006). Deletion of the TREK-1 gene (also called kcnk2) results in a depression-resistant phenotype that mimics treatment with antidepressants (Heurteaux et al., 2006). Interestingly, the Star*D study has identified an association between the existence of four genetic variants (SNPs) in the TREK-1 locus, and a resistance to multiple antidepressant classes (Perlis et al., 2008; Dillon et al., 2010). All these findings indicate that 1) genetic variations in TREK-1 may identify individuals at risk for depression treatment resistance and 2) a search of selective blockers of TREK-1, hitherto not available, might potentially lead to a new generation of antidepressants.
 |
Figure 1. The two pore domain potassium channel TREK1 and the sortilin receptor : A) Activation and Inhibition of TREK-1 and involvement of TREK-1 in cerebral pathologies; B) Cleavage of the mature ligand-binding receptor by furin and release of the propeptide (PE). C) Identification of spadin sequence. |
Growing evidence indicates that trafficking and addressing as well as functional properties of native ion channels depend on their lipidic and proteic environments. K+ channels are known to interact with partner proteins that are crucial for their regulation. In search of protein partners, we identified the sortilin receptor (Mazella et al., 1998; Nielsen et al., 2001) in the regulation of the channel function (figure 1B). Sortilin is a 95-100-kDa type-1 membrane protein, consisting of a large luminal domain, a single transmembrane segment and a short C-terminal cytoplasmic tail. A large part of sortilin is localized at the level of the Golgi apparatus where the protein triggers intracellular functions of trafficking. Depending on its cellular location, sortilin may also act as a receptor or a co-receptor and binds neurotensin (NT), the precursor of the Nerve Growth Factor (proNGF), the receptor-associated protein (RAP), the lipoprotein lipase and the propeptide released from its precursor form (Mazella et al., 1998; Nykjaer et al., 2004). Sortilin as well as TREK-1 are highly expressed in cerebral structures involved in the pathophysiology of depression, such as prefrontal and cingulate cortice, amygdala, hippocampus, nucleus accumbens, dorsal raphe and hypothalamus. Sortilin is synthesized as a proform (prosortilin) which, in late Golgi compartments, is converted to the functional ligand-binding receptor by cleavage and release of a 44 residue N-terminal propeptide (Gln1-Arg44, propeptide) by furin (Munck Petersen et al., 1999). Propeptide binds to the mature receptor with a high affinity (Kd ∼ 5 nM). Structure-function relationship studies have identified that the peptide Gln1-Arg28 was as efficient on the binding activity as the entire propeptide Gln1-Arg44, whereas the affinity of the peptide Gln1-Arg16 was very low (Westergaard et al., 2004) (figure 1C). Therefore, we designed the peptide spadin by conserving the sequence 17-28 in which we added the sequence 12-16 (APLRP) in order to maintain conformational stress. This partial propeptide (Ala12-Arg28) called spadin (figure 1C) was tested for its potential effects on TREK-1 channel regulation and for its validation as antidepressant drug in five behavioral models of depression (Mazella et al., 2010).
The first result of this work is the identification of the NTSR3/Sortilin receptor as a novel TREK-1 partner protein ( figure 2 ). It interacts physically and functionally with TREK-1 to modify its cell surface expression. Experiments of immunoprecipitation of TREK-1 and Sortilin showed that each antibody immunoprecipitated the tested partner, i.e. sortilin precipitated with the TREK-1 antiserum, and TREK-1 with the anti-sortilin antibody ( figure 2 Co-immunoprecipitation), in both COS-7 cells and cortical neurons co-expressing both proteins. The expression of TREK-1 within the plasma membranes, measured either by preparing purified plasma membranes or by using cell surface biotinylation, was enhanced (by a factor 3 and 6, respectively) when COS-7 cells were cotransfected with sortilin ( figure 2 Addressing), confirming the interaction between the two proteins, at least during the channel sorting. Spadin displays identical binding and functional properties as those of the full length propeptide on the neurotensin system. Competition experiments between 125I-labelled spadin and unlabelled spadin or NT on membrane homogenates from TREK-1 transfected COS-7 cells showed that spadin bound specifically to TREK-1 with an affinity of about 10 nM. This binding was selective since NT was unable to displace the binding of 125I-spadin ( figure 2 Binding). Association kinetics of 125I-labelled spadin on whole COS-7 cells expressing TREK-1 showed that the radioactivity associated with cells reached a plateau within 30 min. Removal of surface-bound radioactivity by acid-NaCl wash revealed that about 80% of total 125I-labelled spadin bound at this time was intracellular, indicating that spadin was internalized with TREK-1 following interaction ( figure 2 Internalization). These data strongly suggest that sortilin constitutes a sorting partner of the TREK-1 channel.
 |
Figure 2. Sortilin and Spadin interact with the TREK-1 channel. Co-immunoprecipitation: Immunoprecipitation of Sortilin with anti-TREK-1 antibody (IP α-TREK-1) or immunoprecipitation of TREK-1 with anti- Sortilin antibody (IP α-Sort) from transfected COS-7 cells or mouse cortical neurons. Immunoprecipitated proteins are subjected to Western blots and revealed using anti-sortilin (WB: α-Sort) or anti-TREK-1 (WB: α-TREK-1). Addressing: Influence of Sortilin on the expression of TREK-1 at the plasma membranes. COS-7 cells are transfected with TREK-1 in the absence or in the presence of Sortilin. Crude homogenates, purified plasma membrane proteins or cell surface biotinylated proteins are subjected to Western blot analysis and revealed using anti-TREK-1 antibody. Binding: Competition between 125I–Spadin and unlabeled Spadin (white circles) or NT (black circles) for binding to TREK-1 transfected COS-7 cell homogenates. Internalization, Association kinetics of 125I-Spadin binding to COS-7 cells tranfected with TREK-1. At the indicated times, cells are either washed twice with 500 μL of binding buffer (black circles) or treated with 500 μL of acid-NaCl buffer for 2 min (white circles). |
Electrophysiological studies identify a new function for spadin as a peptidic antagonist of the TREK-1 channel. As previously described (Honore, 2007), TREK-1 basal channel activity was strongly and reversibly activated by arachidonic acid (aa, 10 μM), which induced a typical TREK-1 background current, characterized by outward rectification reversed at the predicted value for EK+. Using the whole-cell patch-clamp technique on TREK-1 transfected COS-7 cells, we demonstrated that 100 nM of spadin were able to block 63±12% of the TREK-1 current stimulated by aa (figure 3A). A spadin dose-response experiment indicated an IC50 value of 70.7 nM at 0 mV (figure 3C). Spadin displayed a better affinity than the propeptide, since 500 nM of propeptide were necessary to block 41 ± 5% of the aa stimulated TREK–1 current measured at 0 mV (figure 3B). Spadin also blocked the TREK–1 activity in CA3 hippocampal neurons on brain slices of wild-type mice and not in kcnk2-/- mice, suggesting a specific inhibitory effect of spadin on the TREK-1 channel (figure 3D).
 |
Figure 3. Effects of Spadin on the TREK-1 channel activity. A-B) Whole-cell currents measured in COS-7 transfected cells in presence of potassium blockers (K+ blockers, 10 mM tetraethyl ammonium (TEA), 3 mM 4-aminopyridine (4-AP), 50 nM charybdotoxin, 10 μM glibenclamide, 100 nM apamin). Cells are clamped at -80 mV and voltage changes are applied by ramp from -100 to 50 mV, 1 s in duration Currents are recorded after TREK-1 activation by 10 μM arachidonic acid (aa) and aa + Spadin (100 nM) (A) or aa + propeptide (PE, 500 nM) (B). Native currents are recorded in the absence (Control) and in the presence of spadin or PE. C) Dose-dependent spadin inhibition of TREK-1 currents, IC50 value at 0 mV is of 70.7 nM. Currents are measured in the presence of 10 μM aa. D, Native currents recorded in the presence of K+ blockers after stimulation by 10 μM of aa on CA3 pyramidal neurons from hippocampus slices in wild-type mice (D) or in kcnk2 deficient mice (TREK-1 KO) (Inset) in the presence or the absence of spadin (1 μM). Currents are elicited by a ramp from –100 mV to 50 mV. |
Finally, we point out spadin as the first peptidic and fast-acting antidepressant. Spadin efficacy was first assessed in the forced swimming test (FST), which is a highly reliable predictor for antidepressant potential (Krishnan and Nestler, 2008). Spadin was administered 30 min before the test by intracerebroventricular (i.c.v.), intravenous (i.v.) or intraperitoneal (i.p.) route at doses of 10−4 to 10−8 M. When placed in an inescapable cylinder of water, spadin-treated mice exhibited reduced floating or immobility times in the three modes of injection with respect to their saline-treated counterparts (figure 4A). The immobility is interpreted as “a state of despair”. The dose-responses of spadin showed that the highest reduced immobility times were observed at the dose of 10−7 M in i.c.v. (66.8%), 10−6 M in i.v. (62.9%) and 10−5 M in i.p. (55.30%) administration. The magnitude of the antidepressant behavior was similar to that observed in fluoxetine-treated wild-type and saline-injected kcnk2-/- mice. It has been proposed that a direct facilitation of 5-HT firing rate in the dorsal raphe nucleus (DRN) should be a requirement for a faster onset of antidepressant action (Blier, 2001). Interestingly, in kcnk2-/- mice we observed an increase in the firing activity of DRN 5-HT neurons (Heurteaux et al., 2006). Obviously, such results raise the possibility that spadin could exert a rapid onset of action. A 4-day treatment with spadin (i.v.,10−6M) significantly reduced the time spent immobile by 43.2% in FST (data not shown). In contrast, subchronic fluoxetine treatment had no effect when compared with saline (data not shown).. The Novelty Suppressed Feeding test (NSF) is usually carried out for demonstrating antidepressant efficacy after chronic, but not acute treatment (Santarelli et al., 2003). Mice treated with spadin (i.v., 10−6 M) for 4 days showed a significant decrease in latency to feed relative to saline-injected animals (figure 4B). A 4-day regimen with fluoxetine (i.p., 3 mg/kg) had no effect in the same conditions (figure 4B). In comparison, a chronic antidepressant treatment with fluoxetine during 21 days in wild-type and kcnk2-/- mice significantly reduced the latency to feed as compared to saline (figure 4B).
 |
Figure 4. Antidepressant effects of Spadin. A) Forced Smimming Test (FST), spadin-treated mice have a shorter time of immobility comparable to that obtained with TREK-1 KO or fluoxetine-treated mice, whatever the way of spadin administration: intracerebroventricular (i.c.v), intravenous (i.v) or intraperitoneal (i.p). Acute treatments: Spadin (10−4 to 10−8 M) or Fluoxetine (3 mg/kg) or Saline solutions are injected 30 min before the test in wild-type and TREK-1 KO mice. B) Novelty-Suppressed Feeding Test (NSF): Subchronic treatments: Spadin (10−6 M), Fluoxetine (1 mg/kg) or Saline solutions are i.v. injected in a 100 μL bolus once a day for 4 successive days before the test. In chronic treatments, spadin (10−6 M) and fluoxetine (1 mg/kg) are i.v. injected in a 100 μL bolus once a day for 21 successive days. At the end of the AD treatment, animals are food deprived for one day and then measured for their latency to feed. 4-day treatment with Spadin significantly reduces the latency to feed when compared to saline or fluoxetine treatments. |
The fast-acting antidepressant potential of spadin, observed in FST tests is further confirmed by its ability to activate the transcription factor cAMP response element-binding protein (CREB) and neurogenesis in the adult mouse hippocampus after a subchronic treatment. Indeed, CREB activity and neurogenesis are considered as specific markers of antidepressant action (Nibuya et al., 1996; Santarelli et al., 2003), but have never been observed before 2 weeks of treatment when using classical antidepressants such as SSRIs. Here, we showed that a 4-day chronic treatment with spadin is able to enhance the pCREB/CREB ratio and consequently increases cell division and proliferation in the SGZ (figures 5A-B). Neurogenesis was analyzed by counting the number of progenitor cells that incorporate the DNA synthesis marker 5-bromo-2’deoxyuridine (BrdU) and that are differentiated into mature neurons. Interestingly, a 4-day treatment with spadin (i.p. 10−5M) significantly increased by two-fold the number of BrdU-positive cells with respect to saline conditions (figure 5A). In contrast, a 4-day regimen with fluoxetine had no effect on neurogenesis, but fluoxetine induced a significant increase in the number of BrdU-positive cells when it was administered during 15 days (data not shown). Dual labeling of BrdU and doublecortin (DCX), a specific marker of neuronal precursors revealed that 85.2% of BrdU-labelled cells expressed DCX (data not shown).
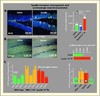 |
Figure 5. Effects of Spadin on neurogenesis, CREB activation and Serotoninergic neurotransmission. A) Spadin increases neurogenesis: (left), Representative photomicrographs of BrdU-labeled neurons in the dentate gyrus of the mouse hippocampus treated either with saline or with spadin (i.v., 10−6 M) for 4 days. (Right) Quantitation of BrdU positive cells of hippocampus treated with saline, fluoxetine or spadin (10−5 M) for 4 days. 85% of BrdU-labeled cells are positive to DCX. B) Enhanced spadin treatment-induced CREB activation in the hippocampus, as assessed by measuring phosphoCREB (pCREB) immunoreactivity. (left), Immunological distribution of pCREB in the mouse hippocampus after a 4-day i.v treatment. pCREB is phosphorylated in the cells near the subgranular zone (SGZ). Right: Quantification of pCREB positive cells/mm2 in hippocampal SGZ. C) Effect of spadin on the firing rate of DRN 5-HT neurons. Spadin (10−5 M in a 100 μL bolus) or its vehicle is i.p. administered. Recordings start 30 min after the injection, and are performed for a maximal duration of 210 min thereafter. Left: Samples of “descents” performed along the DRN, showing typical integrated firing rate histograms in a vehicle-, TREK-1 KO- or spadin-treated animal. Right: 5-HT neuron firing activity, calculated on the basis of all the cells recorded within the successive tracks performed along the DRN. |
As described in kcnk2-/- mice (Heurteaux et al., 2006), spadin leads to an in vivo increase in efficacy of 5-HT neurotransmission as evidenced by an increased firing activity of DRN 5-HT neurons (figure 5C). The facilitation of central 5-HT transmission constitutes the common property of all the antidepressant strategies. From a mechanical point of view, 5-HT1A autoreceptor stimulation reduces DRN 5-HT neuronal firing and, consequently, 5-HT neurotransmission (Blier and de Montigny, 1999). Inhibition of adenylate cyclase and activation of G-protein-coupled inwardly rectifying K+ channels (GIRK) are involved in this negative feedback. The decrease in cAMP concentration (as a result of reduced adenylate cyclase activity) in 5-HT neurons is also thought to induce TREK-1 opening because of a consequent reduction of phosphorylation of Ser333 by PKA (Maingret et al., 2000). According to this model, spadin would induce a depolarization by closing TREK-1 channels and, as described for TREK-1 deficient mice would therefore reduce the negative feedback on 5-HT neurons, resulting in increased 5-HT neurotransmission and in turn in antidepressant-like effects. Direct inhibition of TREK-1 by spadin may also contribute to enhanced 5-HT neuron excitability. Because (i) sortilin is the partner protein of the TREK-1 channel and (ii) both proteins are colocalized in 5-HT-enriched areas known to be involved in the pathophysiology of depression, one may infer that spadin acts predominantly through a modulation of the brain 5-HT circuitry. Nevertheless, we cannot exlude that it can also involve other neurotransmission systems. Whatever the effector pathways involved, the fact that spadin has no effects on kcnk2-/- mice indicates that its action is first and foremost mediated by a modulation of TREK-1 channels.
Conclusion
Spadin can be considered as a natural endogenous antidepressant and constitutes the first peptide identified as an antidepressant with a rapid onset of action. Due to these peculiar properties, spadin brings a new concept to address the treatment of depression. To date, spadin is also the first blocker of TREK-1 channel identified, which is not only of relevance in the field of depression, but also constitutes a useful tool to further understand the role of TREK-1 channels in other neurological pathologies. Because it is possible to dose spadin in the serum of depressed patients spadin could become a marker of depression Detecting and preventing depression certainly could decrease the economic burden of this disease, which is estimated to be 53 billion dollars per year in the United States.
Acknowledgments
This work was supported by the Centre National de la Recherche Scientifique (CNRS), the Agence Nationale de la Recherche (ANR) and the Fondation pour la Recherche Médicale (FRM).
References
- Alloui A, Zimmermann K, Mamet J, et al. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J 2006 ; 25 : 2368–2376. [CrossRef] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Serotonin and drug-induced therapeutic responses in major depression, obsessive-compulsive and panic disorders. Neuropsychopharmacology 1999 ; 21 : 91S–98S. [PubMed] [Google Scholar]
- Blier P. Possible neurobiological mechanisms underlying faster onset of antidepressant action. J Clin Psychiatry 2001 ; 62 (Suppl. 4): 7–11; discussion 37-40. [PubMed] [Google Scholar]
- Blondeau N, Lauritzen I, Widmann C, Lazdunski M, Heurteaux C. A potent protective role of lysophospholipids against global cerebral ischemia and glutamate excitotoxicity in neuronal cultures. J Cereb Blood Flow Metab 2002 ; 22 : 821–834. [CrossRef] [PubMed] [Google Scholar]
- Blondeau N, Widmann C, Lazdunski M, Heurteaux C. Polyunsaturated fatty acids induce ischemic and epileptic tolerance. Neuroscience 2002 ; 109 : 231–241. [CrossRef] [PubMed] [Google Scholar]
- Dillon DG, Bogdan R, Fagerness J, Holmes AJ, Perlis RH, Pizzagalli DA. Variation in TREK1 gene linked to depression-resistant phenotype is associated with potentiated neural responses to rewards in humans. Hum Brain Map 2010 ; 31 : 210–221. [Google Scholar]
- Ettaiche M, Fillacier K, Widmann C, Heurteaux C, Lazdunski M. Riluzole improves functional recovery after ischemia in the rat retina. Invest Ophthalmol Vis Sci 1999 ; 40 : 729–736. [PubMed] [Google Scholar]
- Heurteaux C, Guy N, Laigle C, et al. TREK-1, a K(+) channel involved in neuroprotection and general anesthesia. EMBO J 2004 ; 23 : 2684–2695. [CrossRef] [PubMed] [Google Scholar]
- Heurteaux C, Lucas G, Guy N, et al. Deletion of TREK-1, a background potassium channel, results in a depression-resistant phenotype. Nature Neurosci 2006 ; 9 : 1134–1141. [CrossRef] [PubMed] [Google Scholar]
- Honore E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci 2007 ; 8 : 251–261. [CrossRef] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005 ; 62 : 593–602. [CrossRef] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature 2008 ; 455 : 894–902. [CrossRef] [PubMed] [Google Scholar]
- Lang-Lazdunski L, Blondeau N, Jarretou G, Lazdunski M, Heurteaux C. Linolenic acid prevents neuronal cell death and paraplegia after transient spinal cord ischemia in rats. J Vasc Surg 2003 ; 38 : 564–575. [CrossRef] [PubMed] [Google Scholar]
- Lang-Lazdunski L, Heurteaux C, Vaillant N, Widmann C, Lazdunski M. Riluzole prevents ischemic spinal cord injury caused by aortic crossclamping. J Thorac Cardiovasc Surg 1999 ; 117 : 881–889. [CrossRef] [PubMed] [Google Scholar]
- Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M. Polyunsaturated fatty acids are potent neuroprotectors. EMBO J 2000 ; 19 : 1784–1793. [CrossRef] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two pore domain potassium channels. Am J Physiol 2000 ; 279 : 793–801. [Google Scholar]
- Maingret F, Lauritzen I, Patel AJ, et al. TREK-1 is a heat-activated background K+ channel. EMBO J 2000 ; 19 : 2483–2491. [CrossRef] [PubMed] [Google Scholar]
- Mazella J, Zsurger N, Navarro V, et al. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J Biol Chem 1998 ; 273 : 26273–26276. [CrossRef] [PubMed] [Google Scholar]
- Mazella J, Petrault O, Lucas G, et al. Spadin, a sortilin-derived peptide, targeting rodent TREK-1 channels: a new concept in the antidepressant drug design. PLoS Biol 2010 ; 8 : e1000355. [CrossRef] [PubMed] [Google Scholar]
- Munck Petersen C, Nielsen MS, Jacobsen C, et al. Propeptide cleavage conditions sortilin/neurotensin receptor-3 for ligand binding. EMBO J 1999 ; 18 : 595–604. [CrossRef] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, et al. Neurobiology of depression. Neuron 2002 ; 34 : 13–25. [CrossRef] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci 1996 ; 16 : 2365–2372. [PubMed] [Google Scholar]
- Nielsen MS, Madsen P, Christensen EI, et al. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J 2001 ; 20 : 2180–2190. [CrossRef] [PubMed] [Google Scholar]
- Noel J, Zimmermann K, Busserolles J, et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J 2009 ; 28 : 1308–1318. [CrossRef] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature 2004 ; 427 : 843–848. [CrossRef] [PubMed] [Google Scholar]
- Perlis RH, Moorjani P, Fagerness J, et al. Pharmacogenetic Analysis of Genes Implicated in Rodent Models of Antidepressant Response: Association of TREK1 and Treatment Resistance in the STAR(*)D Study. Neuropsychopharmacology 2008 ; 33 : 2810–2819. [CrossRef] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003 ; 301 : 805–809. [CrossRef] [PubMed] [Google Scholar]
- Westergaard UB, Sorensen ES, Hermey G, et al. Functional organization of the sortilin Vps10p domain. J Biol Chem 2004 ; 279 : 50221–50229. [CrossRef] [PubMed] [Google Scholar]
To cite this article: Heurteaux C, Mazella J, Borsotto M. Spadin, a Sortilin-derived peptide: a new concept in the antidepressant drug design. OCL 2011; 18(4): 202–07. doi : 10.1051/ocl.2011.0395
All Figures
 |
Figure 1. The two pore domain potassium channel TREK1 and the sortilin receptor : A) Activation and Inhibition of TREK-1 and involvement of TREK-1 in cerebral pathologies; B) Cleavage of the mature ligand-binding receptor by furin and release of the propeptide (PE). C) Identification of spadin sequence. |
| In the text | |
 |
Figure 2. Sortilin and Spadin interact with the TREK-1 channel. Co-immunoprecipitation: Immunoprecipitation of Sortilin with anti-TREK-1 antibody (IP α-TREK-1) or immunoprecipitation of TREK-1 with anti- Sortilin antibody (IP α-Sort) from transfected COS-7 cells or mouse cortical neurons. Immunoprecipitated proteins are subjected to Western blots and revealed using anti-sortilin (WB: α-Sort) or anti-TREK-1 (WB: α-TREK-1). Addressing: Influence of Sortilin on the expression of TREK-1 at the plasma membranes. COS-7 cells are transfected with TREK-1 in the absence or in the presence of Sortilin. Crude homogenates, purified plasma membrane proteins or cell surface biotinylated proteins are subjected to Western blot analysis and revealed using anti-TREK-1 antibody. Binding: Competition between 125I–Spadin and unlabeled Spadin (white circles) or NT (black circles) for binding to TREK-1 transfected COS-7 cell homogenates. Internalization, Association kinetics of 125I-Spadin binding to COS-7 cells tranfected with TREK-1. At the indicated times, cells are either washed twice with 500 μL of binding buffer (black circles) or treated with 500 μL of acid-NaCl buffer for 2 min (white circles). |
| In the text | |
 |
Figure 3. Effects of Spadin on the TREK-1 channel activity. A-B) Whole-cell currents measured in COS-7 transfected cells in presence of potassium blockers (K+ blockers, 10 mM tetraethyl ammonium (TEA), 3 mM 4-aminopyridine (4-AP), 50 nM charybdotoxin, 10 μM glibenclamide, 100 nM apamin). Cells are clamped at -80 mV and voltage changes are applied by ramp from -100 to 50 mV, 1 s in duration Currents are recorded after TREK-1 activation by 10 μM arachidonic acid (aa) and aa + Spadin (100 nM) (A) or aa + propeptide (PE, 500 nM) (B). Native currents are recorded in the absence (Control) and in the presence of spadin or PE. C) Dose-dependent spadin inhibition of TREK-1 currents, IC50 value at 0 mV is of 70.7 nM. Currents are measured in the presence of 10 μM aa. D, Native currents recorded in the presence of K+ blockers after stimulation by 10 μM of aa on CA3 pyramidal neurons from hippocampus slices in wild-type mice (D) or in kcnk2 deficient mice (TREK-1 KO) (Inset) in the presence or the absence of spadin (1 μM). Currents are elicited by a ramp from –100 mV to 50 mV. |
| In the text | |
 |
Figure 4. Antidepressant effects of Spadin. A) Forced Smimming Test (FST), spadin-treated mice have a shorter time of immobility comparable to that obtained with TREK-1 KO or fluoxetine-treated mice, whatever the way of spadin administration: intracerebroventricular (i.c.v), intravenous (i.v) or intraperitoneal (i.p). Acute treatments: Spadin (10−4 to 10−8 M) or Fluoxetine (3 mg/kg) or Saline solutions are injected 30 min before the test in wild-type and TREK-1 KO mice. B) Novelty-Suppressed Feeding Test (NSF): Subchronic treatments: Spadin (10−6 M), Fluoxetine (1 mg/kg) or Saline solutions are i.v. injected in a 100 μL bolus once a day for 4 successive days before the test. In chronic treatments, spadin (10−6 M) and fluoxetine (1 mg/kg) are i.v. injected in a 100 μL bolus once a day for 21 successive days. At the end of the AD treatment, animals are food deprived for one day and then measured for their latency to feed. 4-day treatment with Spadin significantly reduces the latency to feed when compared to saline or fluoxetine treatments. |
| In the text | |
 |
Figure 5. Effects of Spadin on neurogenesis, CREB activation and Serotoninergic neurotransmission. A) Spadin increases neurogenesis: (left), Representative photomicrographs of BrdU-labeled neurons in the dentate gyrus of the mouse hippocampus treated either with saline or with spadin (i.v., 10−6 M) for 4 days. (Right) Quantitation of BrdU positive cells of hippocampus treated with saline, fluoxetine or spadin (10−5 M) for 4 days. 85% of BrdU-labeled cells are positive to DCX. B) Enhanced spadin treatment-induced CREB activation in the hippocampus, as assessed by measuring phosphoCREB (pCREB) immunoreactivity. (left), Immunological distribution of pCREB in the mouse hippocampus after a 4-day i.v treatment. pCREB is phosphorylated in the cells near the subgranular zone (SGZ). Right: Quantification of pCREB positive cells/mm2 in hippocampal SGZ. C) Effect of spadin on the firing rate of DRN 5-HT neurons. Spadin (10−5 M in a 100 μL bolus) or its vehicle is i.p. administered. Recordings start 30 min after the injection, and are performed for a maximal duration of 210 min thereafter. Left: Samples of “descents” performed along the DRN, showing typical integrated firing rate histograms in a vehicle-, TREK-1 KO- or spadin-treated animal. Right: 5-HT neuron firing activity, calculated on the basis of all the cells recorded within the successive tracks performed along the DRN. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.




