| Issue |
OCL
Volume 32, 2025
|
|
|---|---|---|
| Article Number | 21 | |
| Number of page(s) | 23 | |
| Section | Quality - Food safety | |
| DOI | https://doi.org/10.1051/ocl/2025017 | |
| Published online | 09 July 2025 | |
Research Article
Utilizing phenolic-enriched sunflower Oleogels and Emulgels as fat replacers: impact on burger oxidative stability, texture, and sensory attributes
Utilisation d’oléogels et d’émulgels au tournesol enrichis en composés phénoliques comme substituts de matières grasses : impact sur la stabilité oxydative, la texture et les propriétés sensorielles de burgers
1
Department of Sport health, College of sport Sciencess and Physical Activity, Princess Nourah bint Abdulrahman University, P.O. Box 84428, Riyadh 11671, Saudi Arabia
2
Agricultural Engineering Research Institute, Agricultural Research Center, Dokki, Giza 12611, Egypt
3
Home Economics Department, Faculty of Specific Education, Alexandria University, Alexandria, Egypt
4
Agricultural Research Center, Food Technology Research Institute, Giza 12611, Egypt
5
Home Economics Department, Faculty of Specific Education, Kafrelsheikh University, Kafrelsheikh, Egypt
* Corresponding author: mohamedabdelbasetsalama@gmail.com
Received:
13
December
2024
Accepted:
19
May
2025
This study aimed to develop and characterize sunflower oil–beeswax oleogels and emulgels enriched with onion peel phenolic extract (EMEX) as fat replacers in food products. High-performance liquid chromatography (HPLC) analysis identified quercetin (432.15 μg/g) and kaempferol (295.23 μg/g) as the predominant phenolic compounds in the onion peel extract. The physical properties of sunflower oil oleogels and emulgels were assessed, including gelation time, oil binding capacity (OBC), firmness, and melting temperature. The OBC slightly decreased from 95.17% in OLEO to 87.45% in EMEX, while firmness decreased from 4.26 N to 3.83 N. The incorporation of EMEX enhanced the total phenolic content (5.49 mg GAE/g) and antioxidant activity (DPPH, 38.84%). Hamburgers formulated with these structured lipids underwent sensory evaluation, which revealed improved appearance (8.35) and juiciness (9.0) for the EMEX-based samples. Storage stability at 4 °C for 14 days demonstrated that burgers containing oleogels and emulgels exhibited better moisture retention, reduced firmness loss, and delayed lipid oxidation compared to the control. The inclusion of phenolic-rich emulgels further improved oxidative stability, as indicated by lower peroxide values and TBARS levels. These findings highlight the potential of oleogels and emulgels, particularly those enriched with natural antioxidants, as viable fat replacers that enhance both quality and shelf life in refrigerated meat products.
Résumé
Cette étude visait à développer et à caractériser des oléogels et des émulgels à base d’huile de tournesol et de cire d’abeille, enrichis avec un extrait phénolique de pelures d’oignon (EMEX), en tant que substituts de matières grasses dans les produits alimentaires. L’analyse par chromatographie en phase liquide à haute performance (HPLC) a identifié la quercétine (432,15 µg/g) et le kaempférol (295,23 µg/g) comme les principaux composés phénoliques présents dans l’extrait de pelures d’oignon. Les propriétés physiques des oléogels et émulgels à base d’huile de tournesol ont été évaluées, notamment le temps de gélification, la capacité de rétention d’huile (OBC), la fermeté et la température de fusion. L’OBC a légèrement diminué, passant de 95,17 % dans l’oléogel (OLEO) à 87,45 % dans l’EMEX, tandis que la fermeté a baissé de 4,26 N à 3,83 N. L’incorporation de l’EMEX a permis d’augmenter la teneur totale en composés phénoliques (5,49 mg GAE/g) et l’activité antioxydante (DPPH, 38,84 %). Des hamburgers formulés avec ces lipides structurés ont été soumis à une évaluation sensorielle, qui a révélé une amélioration de l’apparence (8,35) et de la jutosité (9,0) pour les échantillons à base d’EMEX. Un stockage à 4 °C pendant 14 jours a montré que les burgers contenant des oléogels et émulgels conservaient mieux l’humidité, perdaient moins de fermeté et subissaient une oxydation lipidique retardée par rapport au témoin. L’ajout d’émulgels riches en composés phénoliques a encore amélioré la stabilité oxydative, comme l’indiquent les valeurs plus faibles de peroxydes et de TBARS. Ces résultats soulignent le potentiel des oléogels et émulgels, en particulier ceux enrichis en antioxydants naturels, comme substituts de matières grasses efficaces améliorant à la fois la qualité et la durée de conservation des produits carnés réfrigérés.
Key words: Sunflower oil-based oleogel / Emulgel / burger quality / fat replacer / oxidative stability
Mots clés : Oléogel / huile de tournesol / émulgel / burger / substitut de matière grasse / stabilité oxydative
Publisher note: Le résumé et les mots-clés en français ont été ajoutés le 25/07/2025. French abstract and keywords have been added the 25/07/2025.
© H.A. Alamer et al., Published by EDP Sciences, 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Highlights
Emulgels enriched with onion phenolic extract showed a notable increase in total phenolic content and antioxidant activity which improved oxidative stability.
The incorporation of oleogels and emulgels in burger formulations reduced hardness and cohesiveness while increasing springiness and chewiness. Phenolic extract-enriched emulgel further enhanced the sensory attributes, particularly in terms of flavor and juiciness, without compromising texture.
Both oleogel and emulgel-based burgers exhibited lower peroxide and TBARS values compared to the control. The phenolic-enriched emulgel (EMEX) demonstrated the greatest reduction in lipid oxidation, indicating its potential as a fat replacer with antioxidant benefits in meat products.
1 Introduction
In recent years, food systems have undergone significant changes due to innovations in food processing technologies. The growing pace of modern life has led to an increased demand for convenient and cost-effective food products. Consequently, traditional diets centered around whole or minimally processed foods are being increasingly replaced by industrialized and pre-packaged food options in today’s society (Poti et al., 2017).
Saturated fats are widely used in many processed foods because of technological reasons. These fats play a crucial role in providing desirable sensory attributes, such as texture, mouthfeel, and flavor, making their replacement difficult without compromising key qualities and overall eating satisfaction. Consequently, the intake of essential fatty acids and antioxidants, commonly found in traditional diets, has been replaced by unhealthy fats. This shift is strongly associated with various health problems (Martins et al., 2020).
The search for healthier fat alternatives is not new; the hydrogenation process was developed in the early 20th century as a way to replace saturated fats (animal sources) and reduce cholesterol by using healthier oils (vegetable and marine), which are rich in beneficial polyunsaturated fatty acids. This alters the oils’ saturation levels, giving them manufacturing benefits (firmness and plasticity) (Remig et al., 2010). However, despite these technological advantages, hydrogenation introduced trans fatty acids (TFAs), which are now well recognized for their adverse health effects, including increased cardiovascular risk. This has driven regulatory bans and a strong push toward developing safer alternatives.
The liquid form of vegetable oils restricts their use in certain applications, as they lack the same characteristics as solid fats (Marangoni and Garti, 2018). In light of this and within government initiatives aimed at eliminating unhealthy fats from the market, the food industry has witnessed the emergence of new oil structuring techniques, with oleogels leading the scientific exploration. Oil structuring relies on the creation of a gelator network that forms a stable, thermo-reversible viscoelastic structure without altering the oil’s chemical composition. Conventional hydrogenation, previously used to solidify oils, has been associated with the formation of trans fatty acids (TFAs), which are linked to an increased risk of cardiovascular diseases, inflammation, and other metabolic disorders (Souad, 2024). Additionally, partial hydrogenation can lead to changes in flavor and nutritional quality, prompting regulatory bans or restrictions in many countries (Bhandari et al., 2020). Unlike hydrogenation, oleogels offer a promising method for structuring PUFA-rich oils while preserving their nutritional benefits (Martins et al., 2020). Consumer acceptance of oleogels hinges on their ability to replicate the properties of solid fats. Additionally, oleogels can be customized for specific applications, with structural and textural attributes playing a crucial role. Beyond substituting unhealthy fats with beneficial oils, oleogels can be further enhanced by incorporating bioactive compounds into their formulation. This added functionality not only boosts nutritional value but also enhances product appeal by improving stability and extending shelf life (Pinto et al., 2021). Recent research has also highlighted the potential of oleogels to act as carriers for bioactive compounds, thus enabling the development of multifunctional foods with enhanced health benefits.
Despite the growing interest in oleogels, there remains a significant knowledge gap in fully understanding how to optimize their structural and functional properties, particularly in terms of their ability to replicate the properties of solid fats while incorporating bioactive compounds to enhance their health-promoting properties. A key challenge lies in balancing structural integrity with bioactive loading, especially when applying these systems in complex food matrices such as meat products.
Gels are categorized based on the type of liquid phase they contain, such as hydrogels, emulgels, and organogels. When an aqueous solution forms a gel, it is referred to as a hydrogel. If a gel is created from a biphasic mixture, it is termed an emulgel. When an organic solvent is used to form the gel, it is called an organogel (Martins et al., 2018). To differentiate an edible liquid oil gel, the term of oleogel was preferred within the organogel category (Marangoni and Garti, 2018). Emulgels, combining both emulsification and gelation, offer additional flexibility by enabling the incorporation of aqueous and oil phases simultaneously, widening their potential applications in food systems.
Unsaturated fatty acids typically have melting points below 0 °C due to their chemical structure, but by transforming these fatty acids into oleogels using gelators, they can be made to form semi-solid gels at room temperature (Hyatt et al., 2023; Willett and Akoh, 2019). Oleogels can inhibit photooxidation and free radical-induced peroxidation reactions by immobilizing molecules and decreasing molecular collisions (Tian and Acevedo, 2020).
Another less commonly utilized processing technique examined in this study is the creation of emulsion gels (emulgels). While experimentation with emulgels presents unique challenges due to the presence of water in the sample matrix, they offer the same advantages as oleogels but in an emulsion format. These gels provide additional potential for application in food products, cosmetics, and pharmaceuticals (Sato et al., 2014). Emulgels are especially promising for improving water-holding capacity and delivering both lipophilic and hydrophilic bioactive compounds.
Phenolic compounds derived from onion peels offer a promising approach to enhancing the functional and nutritional properties of food systems. Onion peels, often regarded as agricultural waste, are an abundant and sustainable source of bioactive compounds, particularly quercetin and kaempferol, which are recognized for their potent antioxidant activities (Benito-Román et al., 2020; Celano et al., 2021). These phenolics play a crucial role in scavenging free radicals, delaying lipid oxidation, and improving the oxidative stability of food products (Silva et al., 2022). Incorporating phenolic extracts from onion peels into food formulations not only reduces food waste but also aligns with the growing demand for natural and health-promoting additives (Stoica et al., 2023). Moreover, these compounds have demonstrated effectiveness in enhancing sensory attributes such as flavor and appearance while extending product shelf life (Paesa et al., 2022). Utilizing phenolics from onion peels represents an innovative and eco-friendly solution to developing functional ingredients, offering both environmental benefits and improved product quality.
Meat and meat products play a crucial role in diets worldwide, offering substantial protein content and high nutritional value, but their levels of saturated and trans fatty acids can pose health risks. A diet high in saturated fats is associated with an increased likelihood of various health issues. As a result, the meat industry is under pressure to create healthier products alternatives with an enhanced fatty acid profile to safeguard consumer health. One solution lies in the technological improvement of the fatty acid profile by substituting animal fats in meat products with healthier fat options (Manzoor et al., 2022). However, the incorporation of healthier fats, particularly in structured systems like oleogels and emulgels, requires careful evaluation to ensure that desirable texture, mouthfeel, and stability are maintained in the final product.
The aim of this study is to bridge the gap in knowledge by exploring the use of oleogels and emulgels as structured systems to replace unhealthy fats in food products while incorporating antioxidant-rich phenolic compounds from onion peels. This innovative approach not only addresses health concerns by providing healthier fat alternatives but also contributes to reducing food waste, offering a sustainable solution to the growing demand for functional, eco-friendly food ingredients. The study further evaluates the impact of these structured systems on the physicochemical, textural, and thermal properties of the formulations, aiming to provide comprehensive insights into their practical applications in meat product development.
2 Materials and methods
2.1 Materials
Sunflower oil was sourced from Tanta Company of Oils and Soaps, located in Tanta city, Egypt. Onions were obtained from a local market in Kafrelsheikh, Egypt. Beeswax was supplied by Kahlwax Co. (Kalh GmbH & Co., Trittau, Germany). Tween 80 (polysorbate 80; analytical grade, ≥99% purity) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All chemicals, solvents, and standards used were of analytical grade.
2.2 Methods
2.2.1 Phenolic compounds extraction from onion peels
The onion peels were removed and placed in an air oven (Marconi, MA-035/100, Piracicaba, Brazil) at 60 °C for 8 h. Thirty grams of the dried peels were ground using a mill (IKA, A11 BS000, Germany), and the powder was extracted twice using 300 mL of distilled water at room temperature (25 °C) over 48 h. To minimize the degradation of light-sensitive compounds, the extraction was carried out in the dark by covering the containers with aluminum foil. The extracts were filtered, and the filtrates were concentrated with the help of a rotary evaporator (Alshehri et al., 2024). The remaining residues were frozen at −80 °C and later subjected to lyophilization at −40 °C for 48 h to produce a lyophilized powder. The resulting powder was then stored at −20 °C until further use.
2.2.2 Phenolic compounds determination
The quantification of phenolic compounds in onion extracts was carried out using HPLC-DAD. For this analysis, 30 mg of freeze-dried extract was dissolved in 350 μL of methanol and filtered through PTFE syringe filter (0.45 μm). The analysis was performed using HPLC equipped with a photodiode array detector (Waters Div., Milford, MA, USA) and a reversed-phase LC column (250 mm × 4.6 mm, 5 μm) (Torrance, CA, USA) was utilized at 30 °C. The mobile phases consisted of (A) formic acid (0.5%) in water and (B) formic acid (0.5%) in acetonitrile with stable flow rate (0.8 mL/min), and the injection volume was 10 μL (Paesa et al., 2022).
2.2.3 Development and characterization of the oleogels systems
2.2.3.1 Oleogel and emulgels preparation
To prepare the oleogel (OLEO), 9% of beeswax was melted in a glass beaker at 80 °C for 7 min. Sunflower oil was then added, and the mixture was heated to 80 °C for 20 min while stirring at 700 rpm using a magnetic stirrer (Salama et al., 2024). The concentration of 9% beeswax was chosen based on previous studies and preliminary experiments, which indicated that this ratio provides a stable and firm gel structure suitable for food applications, balancing firmness with desirable spreadability (Alshehri et al., 2025; Salama et al., 2024). The oleogel was then allowed to cool to room temperature and stored at 4 °C in the refrigerator until analysis.
For the emulgel (EM), the previously melted 9% of beeswax was combined with the oil. Tween 80 (0.5 g) was dissolved in water (10%), and the two mixtures were emulsified together using an Ultra-Turrax T25 homogenizer (IKA- Staufen, Germany) at 16,000 rpm for 5 minutes. Emulsification was carried out at room temperature (∼25 °C). The flask was covered to prevent water evaporation during the emulsification process.
For the emulgel with phenolic extract (EMEX), onion phenolic lyophilized extract (0.25 g) was mixed with the Tween 80-water solution. This mixture was then emulsified with the oil-beeswax mixture using the homogenizer at 16,000 rpm for 5 minutes (Keskin Uslu and Yılmaz, 2021). The resulting emulsions were left at room temperature overnight to allow for gelation and were then stored at 4 °C in the refrigerator until analysis (Fig. 1A)
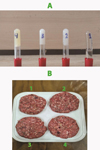 |
Fig. 1 (A1) Sunflower oil, (A2) Oleogel, (A3) Emulgel, (A4) Emulgel with phenolic onion extract; (B1) Control burger, (B2) Oleogel burger, (B3) Emulgel burger, (B4) Emulgel with phenolic onion extract burger. |
2.2.3.2 Gelation time
The samples in glass tubes were fully melted in a water bath at 90 °C and maintained for 2 h to allow for isothermal setting. Afterward, the tubes were removed from the water bath and brought to room temperature, at which point a timer was started. The gelation time was recorded when the tubes were tilted 90° and no flow of the organogel was observed (Yılmaz and Öğütcü, 2015).
2.2.3.3 Oil binding capacity (OBC)
1 mL of sample was transferred into a pre-weighed Eppendorf tube (a) and then placed in a refrigerator at 4 °C for 1 h. After refrigeration, the tube was weighed again (b). The tube was then centrifuged at 9167g for 15 min at room temperature (25 °C). Following centrifugation, the tube was inverted onto a paper cloth to drain any excess liquid oil. After the drainage process, the tube was weighed once more (c) (Yılmaz and Öğütcü, 2015). OBC values were subsequently calculated using the specified equation.
% Released oil = 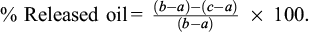
% OBC = 100 – % Released oil
2.2.3.4 Colors determination
Color measurements were taken using a Minolta CR-400 colorimeter (Konica Minolta Sensing, Osaka, Japan) following CIE standards, and the L, a*, and b* values were documented.
2.2.3.5 The centrifuge stability test
The centrifuge stability test was performed by subjecting a 5 g sample in tubes to a centrifugation force of 1300× g for 15 min at room temperature. The stability of the gel was then assessed through visual inspection (Yilmaz and Demirci, 2021).
2.2.3.6 Melting point
In summary, 7 g of molten sample were placed into glass tubes. The tubes were then immersed in a precision water bath set to 38 °C. The water bath, equipped with a temperature control system and digital thermometer, was used to increase the temperature at a constant rate of 1 °C every 3 min. The temperature was carefully monitored and manually adjusted to maintain the desired heating rate until the samples were completely melted and became transparent liquids (Zampouni et al., 2022).
2.2.3.7 Firmness
The firmness of the gels was assessed using a Texture Analyser (CT3 4500, Brookfield, USA) at room temperature. The samples (30 g) were stored in a 50 mL glass beaker in a refrigerator at 5 °C for 24 h to simulate typical storage conditions and ensure uniform cooling and stabilization of the gels. Before testing, each sample was allowed to equilibrate to room temperature for approximately 10 minutes to prevent temperature fluctuation during the measurement. For the test, each sample was removed individually, and the TA4/1000 probe, with a diameter of 1 cm, was used. The firmness was determined by recording the maximum penetration force (Öğütcü and Yılmaz, 2015).
2.2.3.8 Phenolic extraction from oleogel and emulgels
In this extraction procedure, 50 g of different samples were processed through a glass column packed with silica gel (60-Å pore diameter). The column is initially conditioned with a hexane and methanol (1:1 V:V) mixture and subsequently washed with hexane and ethyl acetate (9:1 V:V) to prepare it for sample introduction. The oil sample, dissolved in hexane, is introduced into the column, where the phenolic fraction is extracted into methanol. After passing through the column, the extract is collected and the solvent is removed under vacuum at 40 °C to yield a concentrated extract. These extracts are stored at −20 °C after flushing with nitrogen to prevent oxidation (Steel et al., 2005).
2.2.3.9 Total phenolic content (TPC)
100 μL of samples (oleogel (OLEO), emulgel (EM), and emulgel with phenolic extract (EMEX)) and standards (gallic acid) were added to 750 μL of Folin’s reagent and 750 μL of 7% Na2CO3 and incubated for 45 min at room temperature in dark. The absorbance was then read at 765 nm using spectrophotometer (Analytik Jena Specord 250). The concentration was measured using gallic acid as standard and the results were expressed as mL gallic acid equivalents (GAE)/g sample (Attard, 2013).
2.2.3.10 Antioxidant activity using DPPH assay
0.1 mL of samples (oleogel (OLEO), emulgel (EM), and emulgel with phenolic extract (EMEX)) were mixture with 0.2 mM DPPH solution (2 mL). A control was also performed simultaneously. In the dark, the samples were kept for 30 min. After that, at 517 nm the absorbance was measured using a spectrophotometer (Analytik Jena Specord 250) (Boly et al., 2016). The DPPH radical scavenging activity was determined using the provided equation.
DPPH antioxidant activity (%) = ((control ab − sample ab)/(control ab)) × 100
where: control ab represented the absorbance of the DPPH working solution without the sample, and sample ab corresponded to the absorbance of the DPPH working solution when mixed with samples.
2.2.3.11 Fatty acids measurement
A drop of the oil sample was dissolved in 1 mL of n-heptane. Next, 50 mg of sodium methylate (Merck, Darmstadt, Germany) was added to the tube, which was then agitated for 60 s at room temperature. After adding 100 mL of water, the tube was centrifuged at 3000×g for 5 min, and the lower aqueous phase was carefully removed. Subsequently, 50 mL of HCl (1 mol with methyl orange; Merck, Darmstadt, Germany) was added, and the lower phase was eliminated after brief mixing. Sodium hydrogen sulphate (20 mg) (monohydrate, extra pure; Merck, Darmstadt, Germany) was added to the tube, and the sample was centrifuged at 3000 g for 5 min. A gas chromatograph (GC) with a CP-Sil 88 capillary column (ID 0.25 mm, 100 m long, 0.2 mm film thickness) was used to inject the top phase (n-heptane) that had been collected in a vial. The GC was manufactured by Agilent Technologies Sales & Services GmbH & Co. KG in Waldbronn, Germany and was designated as HP5890. The temperature program started at 155 °C and increased to 220 °C at a rate of 1.5 °C/min, with an injector temperature of 250 °C, a 10-minute isotherm at 220 °C, and a detector temperature of 250 °C. Hydrogen was used as the carrier gas at 36 cm/s, with a split ratio of 1:50. The flow rates of the detector gases were 30 mL/min for hydrogen, 300 mL/min for air, and 30 mL/min for nitrogen. Under 1 milliliter of fluid was injected manually. Fatty acid methyl esters (FAME) percentages were computed as a weight percent using direct internal normalization, and peak areas were estimated using integration software (Salama et al., 2020).
2.2.3.12 Microstructural analysis by scanning electron microscopy (SEM) and atomic force microscopy (AFM)
The microstructure of the oleogel and bigel samples was characterized using both scanning electron microscopy (SEM) and atomic force microscopy (AFM).
For SEM analysis, oleogel samples were rapidly frozen to stabilize the gel network and minimize oil migration. The frozen samples were carefully mounted onto aluminum stubs and immediately coated with a 10 nm gold layer using a Sputter Coater 108 Auto (Cressington Scientific Instruments, Watford, UK) to enhance conductivity. Imaging was conducted using an EVO 40XVP SEM (Carl Zeiss, Milan, Italy), operated under high-vacuum conditions at room temperature with an accelerating voltage of 20 kV. Care was taken to minimize beam exposure time to reduce the risk of thermal damage or structural changes. Images were captured and processed using SmartSEM v. 5.09 software (Carl Zeiss, Milan, Italy).
For AFM analysis, the surface topography of freshly prepared oleogel films was examined using a NanoScope MultiMode AFM (Veeco Metrology, Inc., Santa Barbara, USA). Samples were analyzed in tapping mode to minimize tip-sample interaction forces, thus reducing potential deformation of the soft gel matrix. The instrument was equipped with a standard scanning probe microscopy (SPM) probe, and images were obtained at room temperature shortly after sample preparation to preserve the native microstructure (Abdin et al., 2024).
2.2.3.13 Differential scanning calorimetry (DSC)
The thermal properties, specifically the melting behavior of the oleogel and emulgel samples, were analyzed using differential scanning calorimetry (DSC 200, NETZSCH, Germany) following the method described by Tabibazar et al., 2020 with minor modifications. In summary, 2–4 mg of the sample was placed in an aluminum pan, sealed, and heated from 30 to 200 °C at a rate of 5 °C/min under a nitrogen flow of 20 mL/min.
2.2.3.14 Viscosity of oleogel and emulgels
The viscosity of the samples (gel A and gel B) was determined using a Viscometer (LR Lamy Rheology instrument). The samples were first melted in a water bath at 80 °C. Once fully melted, the oleogel samples were transferred to the viscometer’s sample cell, and their viscosity was recorded as the temperature gradually decreased from 80 °C to 20 °C at a rate of approximately 1 °C per minute. The cooling rate was controlled using the viscometer’s integrated temperature control system, which was programmed to achieve a linear cooling profile. The viscosity values were then plotted against the corresponding temperatures (Sahu et al., 2020).
2.2.4 Chemical properties of oleogel systems and burger
2.2.4.1 Free fatty acid and peroxide value
Free fatty acid and peroxide value were determined according to (AOCS, 2017).
2.2.4.2 Sensory evaluation
The sensory evaluation of the oleogel and burger samples were done by 20 panelists from the Staff of Food Technology Research Institute, Egypt. The acceptance attributes for oleogels were appearance, spreadability, taste, odor and overall acceptability. While for burger samples the attributes were color, odor, flavor, appearance, juiciness, firmness and overall acceptability (Meilgaard et al., 1999).
2.2.5 Development and characterization of burger samples
2.2.5.1 Burger preparation and storage
Lean beef meat was obtained from a local market, Kafrelsheikh, Egypt. Three different hamburger batches were manufactured:
Control burger: with 90% of lean beef and 10% of bovine back fat.
OLEO burger: with 90% of lean beef and 10% of oleogel.
EM burger: with 90% of lean beef and 10% of emulgel.
EMEX burger: with 90% of lean beef and 10% of emulgel with onion peels pheolic extract.
The various ingredients of the burger were individually ground using a domestic MOULINEX HV8 PRO ME686832 meat grinder fitted with a medium coarse grinding plate (4 mm). The ground components were then combined with marine salt (0.5%). Portions of 200 g from each mixture were manually shaped into round patties with a diameter of 9.5 cm and pressed to a height of 2 cm. The burgers were stored at −20 °C in polypropylene bags in triplicate for 14 days. At each storage time point, the samples were analyzed accordingly (Fig. 1B).
2.2.5.2 Texture analyses
A texture analyzer (Instron 1011, Norwood, MA, USA), fitted with a 50-N load cell, was used to assess the texture of the samples. Glass tubes with an internal diameter of 2 cm were filled with 15 mL of the samples and stored at 4 °C. The penetration force was determined by plunging a cylindrical probe into the samples at a penetration speed of 100 mm/min to a depth of 2 cm. The maximum force recorded was reported as the penetration force (N) (Hayes et al., 2005).
2.2.5.3 Weight loss (%)
The weight loss of burger samples was determined by measuring the change in weight over a 14-day storage period at 4 °C. Initially, the raw burger samples were weighed using an analytical balance (accurate to 0.01 g) before storage. The samples were then stored in a refrigerator at 4 °C for 14 days. At each time point (e.g., Days 0, 7, and 14), the burger samples were removed from the refrigerator, allowed to reach room temperature, and weighed again to determine the final weight. The weight loss was calculated as the percentage of weight reduction using the formula:
Weight loss (%) = ((Initial weight − Final weight)/Initial weight) × 100.
2.2.5.4 Cooking loss (CL)
Cooking loss of samples was calculated as described by Erdogdu et al., (2007) following the formula:
Cooking Loss (CL %) = (Mi − Mf)/Mi × 100
Where: Mi = Initial mass of the raw sample in gram, Mf = Final mass of the cooked sample in grams.
2.2.5.5 Water holding capacity
Water holding capacity was determined as described by Jia et al. (2018) with some modifications. In summary, each sample (1 g) mixed with 10 mL of distilled water in centrifuge tube, vortexed for 5 minutes and then centrifuged for 30 min at approximately 2795×g. The removal of water layer was done then, the weights (wt.) of centrifuge tubes were measured and the following equation was used to calculate WHC:
% WHC = [(wt. of tube after decanting (g) − wt. of dry tube (g)) − wt. of total sample (g)] × 100/ wt. of total sample (g).
2.2.5.6 pH measurement
The pH of the burger samples was measured using a pH meter (MP512-03, Hangzhou Tuqi Instrument Co., Ltd, China). A mixture was prepared by blending 5 g of the sample with 45 mL of distilled water using an Ultra-Turrax T25 homogenizer (IKA-Labortechnik, Staufen, Germany) for 30 s at 3000 rpm. Prior to sample analysis, the pH meter was calibrated using buffer solutions at pH 4.0 and pH 6.8 (at 20 °C) following the method outlined by Holman et al. (2017).
2.2.5.7 TBARS determination
Lipid oxidation in the burger samples during different stages of frozen storage was evaluated by measuring TBARS, following the method described by Maraschiello et al. (1999) with minor modifications. In short, 5 g of burger samples were soaked in 20 mL of distilled water and homogenized using an Ultra-Turrax T25 (IKA-Labortechnik, Staufen, Germany) for 10 seconds at 13,500 rpm. The homogenized mixture was combined with 50 mL of cold 7.5% trichloroacetic acid (TCA) solution containing 1 g of EDTA and stirred gently at 50 °C for 30 min. The mixture was then filtered, and the supernatant was collected. A 5 mL aliquot of the supernatant was mixed with 5 mL of 0.02 M 2-thiobarbituric acid (TBA), and the mixture was incubated in a screw-capped tube in a 90 °C water bath for 30 min. TBARS values were determined in triplicate using a spectrophotometer (Analytik Jena Specord 250) at 532 nm against a blank. Calibration curves, based on 1,1,3,3-tetraethoxypropane, were created using the same TBA procedure for sample analysis. Results were expressed in μg MDA/kg of the sample, with all measurements performed in triplicate.
2.2.6 Statistical analysis
The data were analyzed using SPSS software (16.0) to assess variance through the one-way analysis of variance (ANOVA) method. Samples were computed based on three repetitions.
3 Results and discussion
3.1 Phenolic compounds of onion peels extract
The HPLC analysis of the onion peel water extract revealed a rich profile of phenolic compounds, with quercetin (432.15 μg/g) and kaempferol (295.23 μg/g) being the most abundant (Tab. 1). These results allowed us to verify that onion peel extract is a valuable source of bioactive compounds, consistent with previous studies, such as (Benito-Román et al., 2020; Paesa et al., 2022), which also identified quercetin as a dominant flavonoid in onion peels. Although it is well-known that onion peels contain these compounds, the high levels of quercetin and other significant compounds, including gallic acid (154.87 μg/g), caffeic acid (90.45 μg/g), and ferulic acid (72.33 μg/g), contribute to the extract’s potent antioxidant capacity, aligning with findings by Celano et al. (2021). The presence of these phenolics enhances the extract’s potential use as a natural antioxidant in food preservation and nutraceuticals. This study confirms the viability of onion peels, traditionally considered waste, as a sustainable source for bioactive compound extraction, offering both environmental and economic benefits (Kumar et al., 2022). Our findings further highlight the significant potential of onion peel extracts as a functional ingredient in food systems, particularly for antioxidant applications.
Phenolic compounds identified in onion peel water extract (μg/g).
3.2 Development and characterization of the oleogels systems
3.2.1 Physical properties of sunflower oil-beeswax oleogel and emulgel
The data presented in Figure 2A provide insights into the physico-chemical properties of the sunflower oil, oleogel, and emulgel samples. Gelation time reflects how quickly the gel network forms, impacting the texture and stability of the final product. In this case, the addition of beeswax to create the sunflower oleogel resulted in a gelation time of 7.31 min, consistent with studies showing that beeswax, as a structuring agent, forms a stable network by solidifying the oil phase (Salama et al., 2024). Beeswax has been widely recognized for its gelation properties, even at low concentrations, as minimal as 0.5 wt%, which results in a well-structured network with excellent oil-binding capabilities (Abdallah et al., 2000). Patel et al. (2014) also reported that oleogels structured with beeswax formed gel networks rapidly due to the crystallization of wax components, leading to quick gelation times.
The gelation time for the emulgel (EM) was slightly longer (8.72 min), which could be attributed to the additional components in the emulsion, such as water and emulsifying agents. The presence of both an oil phase and a water phase in the emulgel slows down the gelation process compared to the simpler oleogel system, as more interactions between phases need to stabilize before a gel network forms. This result is consistent with existing literature, which suggests that emulgels typically exhibit longer gelation times compared to oleogels due to the complexity of their multi-phase systems (Keskin Uslu and Yılmaz, 2021). When phenolic extract was added to the emulgel (EMEX), the gelation time increased slightly further to 8.99 min. Our findings suggest that the incorporation of phenolic compounds into the emulgel system could affect the gelation dynamics, potentially by interacting with the emulsifying agents or the oil droplets, creating a more intricate network. This slight increase in gelation time indicates that the phenolics play a role in modifying the physical properties of the system, although this effect is less pronounced than in other systems.
The gelling mechanism of beeswax is related to the arrangement of n-alkanes or wax esters into microcrystalline platelets, which aggregate to form a three-dimensional network that effectively traps the liquid oil (Martini et al., 2015). The ability of oleogels to retain oil within their matrix provides insights into their stability and the efficiency of the crystal network’s formation (Okuro et al., 2018), offering potential applications in food products (Doan et al., 2016).
Oil Binding Capacity (OBC) serves as a measure of oil loss under extreme conditions. To assess this, the samples were centrifuged to extract the unbound liquid oil. OBC decreased slightly from 95.17% for the oleogel (OLEO) to 89.39% for the emulgel (EM) and further to 87.45% with the phenolic-enriched emulgel (EMEX) (Fig. 2). This decrease is consistent with the literature, where emulgels have a slightly lower oil-binding capacity than oleogels due to their complex multi-phase structure, but the addition of phenolics could further influence the oil retention properties. Waxes are considered highly promising structuring agents because of their ability to retain oil even at low concentrations, which makes them cost-effective and suitable for food applications (Patel, 2015).
In comparison with other studies, our results verify that the oleogel system, in this case, the OLEO formulation, exhibits the highest OBC (Sun et al., 2022), which is consistent with its superior structural integrity as observed in the texture profile analysis. This study confirms that phenolic compounds in the emulgel system may affect its structural properties, but the influence on OBC is less pronounced compared to the oleogel system.
Firmness decreased from 4.26 N in OLEO to 4.02 N in EM and 3.83 N in EMEX. Similar findings were reported for oleogels prepared from rapeseed oil and 5% beeswax (Kupiec et al., 2020). Our results confirm that the addition of water and phenolic compounds in the emulgel system leads to a decrease in firmness, which is expected due to the disruption of the gel structure caused by the water phase and the phenolic interactions.
The melting temperature is a critical quality characteristic in plastic fats, determining their suitability for various applications (Yılmaz and Öğütcü, 2014). The melting points followed a similar trend, with the OLEO having the highest melting temperature at 50.42 °C, followed by the EM (44.44 °C) and EMEX (40.38 °C) (Fig. 2). These findings support the existing knowledge that emulsification and the incorporation of phenolic compounds interfere with the crystallization process, thereby lowering the melting point (Moghtadaei et al., 2018).
Emulsification introduces an oil-water phase separation, which disrupts the crystallization of the gel network, especially in systems containing beeswax. The presence of water in the emulgel system (EM) leads to a less ordered gel structure, resulting in lower melting points compared to the oleogel (OLEO). The oil and water phases in the emulgel system form a more disordered matrix, preventing the wax components from forming a tightly packed crystalline structure, which typically contributes to a higher melting point.
In addition, the incorporation of phenolic compounds in the EMEX system can further disrupt the crystallization process, as the phenolics may interact with the gelator (beeswax), causing additional structural disorder. This interference with the wax crystallization results in a further reduction in the melting temperature of the EMEX system. In comparison, similar results were obtained for sesame oil-beeswax oleogels with varying concentrations, further validating the observed trends in melting temperature (Sivakanthan, 2024; Sivakanthan et al., 2023).
 |
Fig. 2 Physical, color, texture and viscosity properties of sunflower oil-beeswax oleogel and emulgel. OLEO = sunflower oleogel, EM = sunflower emulgel, EMEX = sunflower emulgel with onion peel phenolic extract. |
3.2.2 Color properties of sunflower oil-beeswax oleogel and emulgel
Figure 2B presented the color properties of OLEO, EM, and EMEX. The L* value, representing lightness, ranged from 55.62 for OLEO to 52.34 for EM, while the addition of phenolic extract resulted in a decrease to 50.16.
The lightness of EM was lower than OLEO due to the incorporation of water in the emulsion. This was in agreement with Keskin Uslu and Yılmaz (2021) who reported that as the added water level increased, the L value decreased slightly. However, the introduction of onion extract, which may have darker pigmentation, leads to a reduction in lightness. Onion extract, rich in phenolic compounds, can introduce more color, affecting the overall lightness of the emulgel.
The results for color b showed a gradual increase from OLEO (2.15) to EM (2.56), and EMEX (2.93), indicating an intensified yellow hue as the structure became more complex. This could be attributed to the presence of phenolic extract which increased the b* value, likely due to the yellow-brownish hues of phenolic compounds. These findings are consistent with previous study of Keskin Uslu and Yılmaz (2021) who noted adding water in the emulgels caused more yellowness.
For a, no significant differences were observed between samples. Negative numbers for a also were reported by Keskin Uslu and Yılmaz (2021) for oleogel and emulgel samples.
As all the samples exhibited a creamy-white color, there should be no issues with their use in real-world food or non-food applications.
3.2.3 Chemical properties of sunflower oil-beeswax oleogel and emulgel and total phenolic content (TPC) and antioxidant acticity (DPPH %) compounds extracted from sunflower oil-beeswax oleogel and emulgel
The total phenolic content (TPC) of the sunflower oil (4.15 mg GAE/g), oleogel (OLEO) (4.55 mg GAE/g), and emulgel (EM) (4.35 mg GAE/g) showed similar values, with no statistically significant differences among them (Tab.2). However, when phenolic extract was added to the emulgel (EMEX), TPC significantly increased to 5.49 mg GAE/g, confirming the contribution of the added onion peel extract, which is rich in antioxidants such as quercetin and kaempferol.
DPPH antioxidant activity showed a similar trend, with sunflower oil exhibiting a DPPH value of 29.25%. The activity increased slightly for OLEO (30.23%) and EM (29.88%). However, the addition of the phenolic extract in EMEX resulted in a significant increase in antioxidant activity (38.84%), due to the potent antioxidant properties of the onion peel extract.
Free fatty acid (FFA) content was relatively consistent across all samples, ranging from 2.59% to 2.77%, indicating that the addition of wax, water, or phenolic extracts did not significantly influence the free fatty acid content.
Peroxide values (PV), which measure oxidative rancidity, remained stable across all formulations, ranging from 3.44 to 3.65 meq O2/kg. The lack of significant change in PV can be attributed to the fact that the systems were not subjected to prolonged storage conditions or oxidative stress, meaning no significant oxidation occurred during the experiment. These values are comparable to those reported in the literature for fresh sunflower oil and oleogel systems, which typically range between 2.0 and 5.0 meq O2/kg (Marangoni and Garti, 2018; Hwang, 2020), indicating that the formulations in this study maintained acceptable oxidative stability within expected limits.
Finally, all formulations demonstrated stability under centrifugation, which was evaluated to assess the physical integrity of the systems under mechanical stress. The oleogel (OLEO) and emulgel (EM) systems exhibited no phase separation or significant degradation during centrifugation, indicating that the gel networks formed by the structuring agents, such as beeswax, were resilient to mechanical stress (Zhou et al., 2024). This result suggests that both systems are physically stable and capable of maintaining their structural integrity under conditions that would typically challenge the stability of emulsions and gelled systems. The inclusion of phenolic extract in the EMEX system did not compromise this stability, as no significant changes were observed in the structure or phase distribution following centrifugation. This finding is important, as it suggests that the addition of bioactive compounds, such as the onion peel extract, does not adversely affect the physical stability of the emulgel, making it a promising system for food applications where both structural integrity and antioxidant functionality are required.
Total phenolic content (TPC), antioxidant activity (DPPH) and chemical properties of sunflower oil-beeswax oleogel and emulgel.
3.2.4 The texture properties of sunflower oil-beeswax oleogel and emulgel
The texture properties of the sunflower oil-beeswax oleogel and emulgel are critical in understanding their functionality and suitability for various food applications. The results (Fig. 2C) demonstrated significant variations in hardness, adhesiveness, cohesiveness, and springiness between the systems. The OLEO system exhibited the highest hardness value (3.1), followed by EM (2.9) and EMEX (2.8). The higher hardness of OLEO can be attributed to the solid-like structure of the oleogel, which is created by the formation of a beeswax network. The rigidity of this structure enhances its ability to resist deformation, which is typical of oleogels. On the other hand, emulgels, such as EM and EMEX, contain both oil and water phases stabilized by emulsifiers, resulting in a softer texture due to their higher liquid content and more flexible matrix (Han et al., 2014).
Adhesiveness measures the energy required to break the bond between the product and a surface. The EMEX system demonstrated the highest adhesiveness (0.7 mJ), followed by OLEO (0.6 mJ) and EM (0.5 mJ). Adhesiveness is important for food systems, as it affects how the product interacts with the mouth. The higher adhesiveness in EMEX could be related to the increased molecular interactions in the emulgel matrix with the addition of phenolic extract. The phenolic compounds may act as natural binders, contributing to higher surface stickiness (Tanislav et al., 2022). This observation is important for applications where adhesion to surfaces or controlled release of active compounds is desired.
Cohesiveness reflects the internal strength of the bonds within the product, with higher values indicating a stronger cohesion. The cohesiveness was slightly enhanced in EMEX (0.39), followed by EM (0.38) and OLEO (0.36). The increase in cohesiveness in EMEX could be attributed to the addition of phenolic compounds, which may enhance the gel matrix through non-covalent interactions—such as hydrogen bonding and hydrophobic associations—leading to a more compact and tightly bound structure. Previous studies have noted that cohesiveness is crucial in determining the ability of a system to withstand deformation and recover its original form (Tanislav et al., 2022). The values obtained in this study are consistent with previous research on oleogels, which reported cohesiveness values ranging from 0.05 to 0.21 for various gelators (Tanislav et al., 2022; Lim et al., 2017).
Springiness, or the ability of the sample to return to its original shape after deformation, was measured for all three systems. The results showed similar springiness values: OLEO (2.84 mm), EM (2.92 mm), and EMEX (2.91 mm). These values indicate that all the systems exhibit comparable elasticity, with only slight variations that were not statistically significant. The springiness of the systems was influenced by the matrix structure, with both oleogels and emulgels demonstrating relatively high elasticity, indicative of their ability to recover shape after compression.
In conclusion, the texture properties of the oleogel and emulgel systems are influenced by the gelation structure, the presence of phenolic compounds, and the interactions between the oil, water, and structuring agents. The OLEO system, with its higher hardness, lower adhesiveness, and slightly lower cohesiveness, is indicative of a more rigid, solid-like structure. In contrast, the EM and EMEX systems, with their lower hardness and higher adhesiveness, exhibit more flexible and less rigid structures, with the phenolic extract in EMEX contributing to increased cohesiveness. It is important to note that these textural characteristics are specific to the formulations studied here and may vary with different gelator types, concentrations, or oil phases. These findings highlight the functional and sensory implications of these systems, particularly in applications where textural properties like adhesiveness, cohesiveness, and springiness are important.
 |
Fig. 3 AFM, SEM and DSC of sunflower oil-beeswax oleogel and emulgel. |
 |
Fig. 4 Texture analysis of burger samples. OLEO = sunflower oleogel, EM = sunflower emulgel, EMEX = sunflower emulgel with onion peel phenolic extract. |
3.2.5 Viscosity of sunflower oil-beeswax oleogel and emulgel under different temperature
The viscosity results confirmed the well-known trend that increasing temperature leads to a decrease in viscosity for all systems. At 30 °C, EMEX exhibited the highest viscosity (2041.67 cP), followed by OLEO (2016.67 cP), and EM (1991.67 cP), indicating that the presence of phenolic compounds in EMEX may enhance the gel matrix and network structure (Fig. 2). As temperature increased to 50 °C, the viscosity for all systems decreased, with OLEO showing 1551.28 cP, EM 1422.62 cP, and EMEX 1361.11 cP. The more substantial viscosity drop in the emulgel systems (EM and EMEX) could be attributed to the weakening of the oil-water emulsion structure at higher temperatures (Xia et al., 2022). At 80 °C, OLEO retained the highest viscosity (1196.15 cP), while EM and EMEX exhibited lower viscosities of 1080.36 cP and 1025.85 cP, respectively.
While the decrease in viscosity with increasing temperature is well established, the comparison of the oleogel and emulgel systems in this study provides useful insights into how phenolic compounds influence the thermal stability and viscosity behavior of structured food systems, particularly under conditions where temperature changes are expected. The addition of phenolic extract in EMEX was found to enhance viscosity at lower temperatures, which could contribute to the stability and texture of food products during storage and processing.
3.2.6 Fatty acids composition of sunflower oil-beeswax oleogel and emulgel
Palmitic and stearic acids were the major saturated fatty acids (SFA) in all samples. The amount of SFA remained fairly consistent across sunflower oil, OLEO, and emulgels (Tab 3). The formation of oleogels and emulgels, which involved structuring the oil with beeswax, did not significantly affect the fatty acid profile. Oleic acid was the predominant monounsaturated fatty acid. It showed a slight decrease in OLEO, EM and EMEX compared to the original sunflower oil. Linoleic acid is the most abundant fatty acid across all samples, but its concentration decreases in the oleogel and emulgel formulations. Similarly, the linolenic acid content also slightly decreases. The decrease in oleic and linoleic acids content during the formation of oleogels and emulgels could be due to the processing conditions, including temperature and shear during gelation or emulsification. Research by Moghtadaei et al. (2018) showed that structuring oil with beeswax different concentrations typically retained the original fatty acid composition.
Fatty acids composition of sunflower oil-beeswax oleogel and emulgel.
3.2.7 Sensory evaluation of sunflower oil-beeswax oleogel and emulgel
The sensory evaluation of sunflower oil-based oleogel (OLEO) and emulgel (EM and EMEX) showed notable variations in terms of appearance, spreadability, taste, odor, and overall acceptability (Tab. 4).
For appearance, EMEX received the highest score (8.35), followed by EM (7.83) and OLEO (7.42). The higher appearance score for EMEX can likely be attributed to the presence of phenolic compounds, which could have contributed a subtle sheen or color to the emulgel, enhancing its visual appeal. This observation aligns with previous research, such as Pintado and Cofrades (2020), who found that natural extracts can enhance the appearance of emulsions due to their color-contributing properties. This visual enhancement could make the product more appealing to consumers.
Regarding spreadability, EMEX (8.69) and EM (8.63) scored higher than OLEO (7.85). This difference is primarily due to the structural properties of these systems. Emulgels, with their combination of both oil and water phases, tend to have a smoother, more pliable structure, making them easier to spread. In contrast, oleogels, with their solid-like network formed by the beeswax gelation, tend to be more rigid and less spreadable (Milutinov et al., 2023). Although the addition of phenolic extract in EMEX did not significantly affect spreadability, it maintained the superior spreadability of the emulgel systems compared to the oleogel. This demonstrates the inherent advantage of emulgels in terms of spreadability, making them more consumer-friendly in applications that require easy spreading.
For taste and odor, there were no significant differences between the three samples. This suggests that the structural differences between the oleogel and emulgel systems did not influence the basic sensory characteristics like taste or odor, indicating that these systems may perform similarly in terms of flavor profile and scent.
However, overall acceptability showed significant differences. Both EM (8.88) and EMEX (8.84) were more acceptable than OLEO (8.38). This higher acceptability for emulgels can be attributed to several factors, including their smoother texture, better spreadability, and possibly more pleasing mouthfeel (Yılmaz, 2022). Emulgels, due to the presence of both oil and water phases, likely have a more light, creamy texture compared to oleogels, which are more solid and dense (Pușcaș et al., 2020). The higher acceptability of emulgels, particularly EMEX, could also be influenced by the antioxidant properties provided by the phenolic extract, which may enhance the overall perception of quality. Furthermore, the lighter texture and superior spreadability of emulgels could make them more suitable for a wider variety of consumer applications.
In summary, the sensory evaluation revealed that emulgels (EM and EMEX) were favored over oleogels (OLEO), mainly due to their superior spreadability, smoother texture, and higher overall acceptability. The addition of phenolic extract in EMEX did not significantly affect spreadability but contributed to a higher acceptability score. This suggests that the emulgel system, particularly with the addition of bioactive compounds like onion peel phenolics, offers promising functional and sensory benefits, making it a desirable option for food formulations that prioritize texture and overall consumer satisfaction.
Sensory evaluation of sunflower oil-beeswax oleogel and emulgel.
3.2.8 SEM of sunflower oil-beeswax oleogel and emulgel
The SEM images (Fig. 3A, B, C) showed distinct microstructural differences between OLEO, EM, and EMEX. OLEO exhibited a relatively smooth and homogeneous surface structure with spherical voids or pores, characteristic of a stable oleogel matrix made from sunflower oil and beeswax. The visible round pores suggested uniform gelation, indicating that the beeswax had effectively trapped the sunflower oil within the gel matrix, providing structural integrity and reducing movement of the oil. This stable, uniform matrix in OLEO is consistent with the high hardness value observed for OLEO (3.1), indicating a more rigid, solid-like structure. The gelation network formed by the beeswax matrix likely contributes to OLEO’s superior oil retention capacity (OBC) of 95.17%, as the wax network effectively traps the sunflower oil, preventing oil leakage even under extreme conditions.
For EM, the introduction of water and Tween 80 into the system transformed the structure into a more irregular and disrupted appearance. The presence of both oil and water phases, emulsified using Tween 80, introduced more complexity into the microstructure, with larger, irregularly shaped particles and fragments (Carpintero-Tepole et al., 2017). The surface appeared more disorganized, which was typical for emulsions where oil droplets were dispersed within an aqueous medium. This complexity correlates with the lower hardness values observed for these systems (EM: 2.9, EMEX: 2.8), as the emulsion structure is generally more flexible and less rigid than oleogels. The OBC values for EM (89.39%) and EMEX (87.45%) were also lower than OLEO, likely due to the added water phase, which reduces the oil-binding capacity of the system.
For EMEX, the addition of the phenolic extract further altered the microstructure, as evidenced by the rough, fragmented surface with larger, blocky particles (Ngo et al., 2022). The microstructure appeared more irregular and coarse, with larger aggregates, indicating that the phenolic extract may cause phase separation or aggregation of components within the emulgel. The presence of phenolic compounds in EMEX did not significantly improve the OBC compared to EM, although it did enhance cohesiveness (0.39 in EMEX) and adhesiveness (0.7 mJ), which can be attributed to the interaction between the phenolic extract and the emulgel matrix. These changes in the microstructure are likely responsible for the increase in cohesiveness and adhesiveness in EMEX, further influencing the texture properties observed in the rheological tests (Min et al., 2023).
The oil-binding capacity (OBC) of the systems can be directly linked to the observed microstructural differences in the SEM images. OLEO, with its solid-like network formed by beeswax, exhibited the highest OBC (95.17%), indicating that the rigid, well-structured gel matrix is highly effective at trapping oil. The SEM results support this, showing a stable, uniform matrix with spherical pores, which suggests that the oleogel can maintain its integrity and oil retention even under challenging conditions.
On the other hand, the lower OBC in EM (89.39%) and EMEX (87.45%) corresponds to the more complex microstructure observed in their SEM images, where larger, irregular particles and phase separation were seen. These disruptions in the microstructure could result in a less stable network, thereby reducing the ability of the system to retain oil. While the addition of phenolic compounds in EMEX led to some structural changes (as seen in the rougher, more fragmented surface in SEM images), the impact on OBC was less pronounced, as the complex oil-water phases could limit the gel’s oil-holding capacity.
It should be noted that the SEM images represent the microstructure of the samples after preparation steps, including freezing, drying, and gold coating. These processes may introduce artifacts such as shrinkage, oil migration, or changes in surface texture, which can affect the true native morphology of the oleogel and emulgel systems. Therefore, while the SEM images provide valuable insights into the structural differences between formulations, the interpretations should be considered indicative rather than definitive of the native gel structures.
In summary, the SEM images helped elucidate the microstructural differences between the oleogel and emulgel systems, which in turn are reflected in the texture properties (hardness, cohesiveness, and adhesiveness) and OBC values. The rigid network in OLEO resulted in higher OBC and hardness, while the more flexible and complex structure of EM and EMEX contributed to lower OBC and softer texture. The phenolic extract in EMEX contributed to increased cohesiveness and adhesiveness, as seen in both the SEM images and the texture profile analysis.
3.2.9 AFM of sunflower oil-beeswax oleogel and emulgel
The AFM images of OLEO, EM, and EMEX revealed interesting differences in surface morphology and nanostructural features, which influenced their physical properties and potential applications (Fig. 3D, E, F).
The first image (OLEO) showed a relatively smooth surface with nano-scale aggregates. The uniformity of the surface and slight elevation (up to 1.4 μm) suggested a homogeneous distribution of the beeswax within the sunflower oil, creating a stable network that could enhance the oleogel’s structural integrity and oil-holding capacity. This smooth texture was indicative of good gelation, with small, well-distributed aggregates that contributed to the oleogel’s solid-like properties (Zhang et al., 2023).
The second image (EM) showed a much more uneven surface, with larger and more pronounced peaks and valleys (up to 1.9 μm). The introduction of water and Tween 80 disrupted the homogeneity seen in the oleogel, leading to a more complex structure (Zhang, 2020). The emulgel’s surface suggested increased aggregation and clustering of components, likely due to the emulsification process. The water phase interacted with the oil and beeswax network, causing phase separation and leading to larger nanostructures (Naderizadeh et al., 2019). This indicated the formation of a more complex gel matrix, which could affect its viscosity and stability.
For EMEX showed a rough surface similar to the second, but with more defined pits and irregularities. The addition of extract introduced bioactive compounds, which may further disrupt the emulsion system and contribute to the increased surface irregularity (Boostani et al., 2024). The roughness and variability in texture suggested interactions between the extract’s phenolic compounds and the beeswax-oil matrix, potentially improving the antioxidant properties of the emulgel but also affecting its structural stability. This texture could enhanced bioactive release but may compromise some mechanical stability compared to the simpler emulgel.
3.2.10 DSC of sunflower oil-beeswax oleogel and emulgel
Figure 3G, H, I showed DSC curves of OLEO, EM ad EMEX. The DSC curve for OLEO sample showed a glass transition temperature (Tg) of 44.18 °C, a melting temperature (Tm) of 158.92 °C, and a decomposition temperature (Td) of 249.08 °C. The enthalpy change during melting was also indicated (129.03 J/g), which was a measure of the energy required for phase transitions. These thermal events corresponded to the melting and crystallization behavior of the oleogel, which was influenced by the beeswax structuring the oil (Martins et al., 2017). The thermal stability up to 249 °C indicated that the oleogel could withstand significant heat before breaking down.
In EM, the DSC curve showed a slightly higher glass transition temperature (Tg) of 49.22 °C and a higher melting temperature (Tm) of 173.29 °C compared to the oleogel. The decomposition temperature (Td) is 228.30 °C. The presence of water and the emulsifying agent (Tween 80) likely resulted in a more thermodynamically stable system, as indicated by the higher melting temperature and larger enthalpy (198.71 J/g) compared to the oleogel. However, the slight decrease in Td might suggest a reduced thermal stability due to the emulsification process and added water.
For EMEX, the glass transition temperature (Tg) was observed at 51.14 °C, which may be attributed to the amorphous regions within the emulgel matrix, likely arising from hydrophilic components such as Tween 80 and phenolic extract. The main thermal event observed at approximately 174.82 °C is interpreted not as the melting point of beeswax (typically 60–65 °C) but rather as an indication of thermal degradation or a high-temperature phase transition within the composite system. The onset of decomposition (Td) was noted at 233.16 °C, suggesting that the addition of the phenolic extract slightly enhanced the thermal stability of the emulgel matrix. The extract might be providing additional stability due to its antioxidant properties, which could reduce the rate of thermal degradation (Chaaban et al., 2017). Additionally, the enthalpy (210.12 J/g) was higher than EM, indicating that the extract might contribute to stronger molecular interactions or changes in the crystallization behavior of the emulgel. These results were in agreement with Yaqoob et al. (2024) who reported that DSC results exhibited improved melting points of the multicomponent oleogel.
The DSC curve for the OLEO sample showed a glass transition temperature (Tg) of 44.18 °C, a melting temperature (Tm) of 158.92 °C, and a decomposition temperature (Td) of 249.08 °C. The enthalpy change during melting was also indicated (129.03 J/g), which was a measure of the energy required for phase transitions. These thermal events corresponded to the melting and crystallization behavior of the oleogel system, which includes both beeswax and sunflower oil. It is important to note that the reported Tg and Tm values correspond to the oleogel system, not to pure beeswax, as the combination of beeswax and sunflower oil can alter the thermal properties. The thermal stability up to 249 °C indicated that the oleogel could withstand significant heat before breaking down.
In addition to the oleogels, the DSC analysis for the emulgels (EM and EMEX) showed that the presence of water and emulsifiers such as Tween 80 in EM and the addition of phenolic extract in EMEX had significant effects on their thermal properties. EM exhibited a higher Tm and enthalpy compared to OLEO, suggesting that emulsification leads to a more thermodynamically stable system. The introduction of water and emulsifiers in EM likely disrupted the crystallization behavior of the oleogel network, leading to a lower Td. The addition of phenolic compounds in EMEX increased the Tg and enthalpy, indicating that the phenolics contributed to stronger molecular interactions in the emulgel matrix, further affecting the system’s thermal behavior.
3.3 Development and characterization of burger samples
3.3.1 Physical and chemical properties of prepared burger
The pH values of the burger samples ranged from 6.84 to 7.11, with the control burger having the lowest pH (6.84) and the oleogel burger showing the highest pH (7.11). However, no significant differences in pH were observed between the samples (Tab. 5). These slight variations in pH could be attributed to the incorporation of different systems, such as oleogel and emulgel, into the burger formulations. Despite these small differences, the overall pH remained similar across all samples, and the changes did not lead to any meaningful variations in the overall chemical properties of the burgers.
In general, color plays a significant role in consumers’ decisions to either purchase or reject a package of meat, as it is often associated with the freshness and potential spoilage of the product (Singh et al., 2011). The L* (lightness) values showed that the control, OLEO, and EM burgers had similar lightness values, with no significant differences observed between the samples (Control: 45.62, OLEO: 44.52, and EM: 45.23). The EMEX burger, however, showed a slightly lower L* value of 43.99, indicating a slight darkening of the meat. This decrease in lightness could be attributed to the phenolic compounds in the onion peel extract. Phenolic compounds, such as quercetin and kaempferol, are known to interact with proteins, which could influence the color of the meat, especially under heat treatment (Zhang et al., 2021; Paesa et al., 2022). The darkening effect is likely due to these phenolic compounds, which might have facilitated the Maillard reaction or contributed to the formation of darker pigments within the meat (Zhang et al., 2015).
The a* (redness) and b* (yellowness) values showed minimal variations across the samples, with no significant differences observed between the control, OLEO, EM, and EMEX. This suggests that while slight differences in color were detected, they were not substantial enough to affect the overall appearance of the burger. These findings are consistent with the existing literature, which suggests that phenolic compounds may have a more pronounced effect on lightness (L*) than on the redness (a*) or yellowness (b*) of meat products (Issara et al., 2022).
Color changes in restructured meat products during storage can result from various reactions, including the Maillard reaction, lipid oxidation, and the oxidation of myoglobin (Chaijan et al., 2021). In this study, the phenolic compounds in the EMEX burger could have interacted with the meat’s amino acids, which may have led to the observed color change, particularly during heat treatment.
Additionally, comparing the current findings with other studies using sunflower oil-based structuring systems or similar structuring agents (such as beeswax) would provide a more relevant context. Studies that utilized sunflower oil and beeswax-based structuring agents (Ferdaus et al., 2024) have mentioned similar effects on color.
da Silva et al. (2019) observed a difference in the a* value of the oleogel meat product, but no significant changes were noted for the L* and b* values. Conversely, a study by Heck et al. (2019) reported similar findings to our study regarding the color attributes (excluding L*). They examined low-fat burgers made with a hydro-gelled emulsion containing a mixture of chia and linseed oils.
Additionally, Moghtadaei et al. (2018) discussed the use of sesame oil oleogel, stabilized with beeswax, as a fat substitute in beef burgers at concentrations of 5%, 7.5%, and 10%. Also, they reported that there were no significant differences in the a* and b* values between samples as the proportion of oleogel increased in burger production. While Sadeghinejad et al. (2019) found that L* value decreased in burger samples prepared by phenolic compounds.
The water-holding capacity (WHC) was highest in the control burger (27.21%) and lowest in the oleogel and emulgel burgers, indicating that replacing animal fat with oleogel and emulgel slightly reduced the ability of the meat matrix to retain water. This lower WHC can be attributed to the structural properties of the fat alternatives (Tian et al., 2025). Oleogels and emulgels form more rigid, hydrophobic matrices compared to animal fat, which is more hydrophilic and capable of holding water more effectively (Pinto et al., 2021). Specifically, oleogels are structured by gelators, such as beeswax, which create a solid-like matrix optimized for trapping oils but not as effective in retaining water. This hydrophobic network limits the ability of the system to absorb and hold water, unlike animal fat, which has a higher affinity for water molecules (Jimenez-Colmenero et al., 2015).
Furthermore, the presence of emulsifiers in emulgels introduces an additional layer of complexity. While emulsifiers stabilize oil droplets in the system, they do not maximize water retention, leading to a reduced WHC compared to animal fat. The structural integrity of the emulsion matrix in the emulgels, designed to stabilize both oil and water phases, does not facilitate the retention of large amounts of water, further contributing to the lower WHC observed in these systems (O’Sullivan et al., 2016).
This reduced water retention in both oleogel and emulgel systems can be linked to their inability to form the same type of protein-water interactions found in traditional meat systems, where water is held by both fat and protein components (Asyrul-Izhar et al., 2023). Consequently, the lower WHC observed in the oleogel and emulgel burgers is primarily due to the structural characteristics of these fat alternatives, which do not possess the same water-binding capabilities as animal fat.
Cooking loss was lowest in the control burger (25.84%) and highest in EM ad EMEX burgers (29.63 ad 29.87%, respectively). The higher cooking loss in the emulgel samples may be attributed to the higher moisture content in the emulsion system, leading to greater water release during cooking. This finding is consistent with studies such as that by Pintado and Cofrades (2020), where fat replacement in meat products, particularly using emulgels, led to increased cooking loss due to higher moisture migration during thermal treatment.
pH, color, water holding capacity (WHC) and cooking loss (CL) of burger samples.
3.3.2 Texture analyzer of prepared burger
The texture characteristics of food play a crucial role in assessing the overall quality, especially in meat and meat-related products (de Souza Paglarini et al., 2018; Gómez-Estaca et al., 2019).
The control burger had the highest hardness value (20.15), indicating a firmer texture compared to the oleogel and emulgel samples (Fig. 4). This higher hardness was likely due to the presence of solid animal fat (Fig. 4), which provided structure and firmness. In contrast, the hardness of OLEO (17.52), EM (15.37), and EMEX (15.22) burgers was significantly lower. The reduction in hardness could be attributed to the softer texture of oleogels and emulgels, which lack the solid fat crystals present in animal fat. The hardness of meat products can be attributed to the smaller size of fat globules. During production, these fat globules become encased in salt-soluble proteins, particularly myosin and actin, which create a protein film around the globules. Smaller fat globules require a significantly larger amount of this interfacial protein film. As the formation of the protein film increases, the product’s hardness also rises. Consequently, the presence of larger fat globules may explain the reduction in hardness when animal fat is replaced (Youssef and Barbut, 2009).
The control burger showed the lowest cohesiveness value (0.52), while OLEO (1.12), EM (1.20), and EMEX (1.18) had significantly higher values. Higher cohesiveness in these samples suggested that the fat alternatives better bind the burger matrix, leading to a more cohesive product. This was likely due to the gel-like structure of oleogels and emulgels, which enhanced the binding between protein and water.
The control burger had the highest chewiness value (2.84), which correlated with its higher hardness. Chewiness was reduced in OLEO (1.88), EM (1.79), and EMEX (1.65) burgers, likely due to their softer texture. As hardness decreased, so did the effort required to chew the product.
Springiness was highest in OLEO (2.22), EM (2.19), and EMEX (2.20) burgers (without any significant differences), compared to the control (0.47). This indicated that the fat replacers created a more elastic texture. The gel structure of oleogels and emulgels may have contributed to better recovery after compression, as these systems are known to enhance the elasticity of meat products. Study by Oppong et al. (2022) showed an increase in springiness emulgel formulated nugget I comparison with control nuggets.
Overall, these findings suggested that replacing animal fat with oleogels and emulgels leads to softer, more cohesive, and elastic textures, which may affect the mouthfeel and consumer preference. The addition of onion phenolic extract did not significantly affect the texture attributes compared to the plain emulgel, suggesting its impact was primarily on flavor rather than texture. Similar results were reported by Oppong et al. (2022) who reported that fish nuggets control showed higher hardness than the ones prepared by emulgel.
During the emulsification process, it is important to balance the water immobilized by hydrocolloids with the amount of water available to interact with meat proteins. This balance is key to reducing hardness and enhancing the elasticity of the emulsion-based gel system (Mejia et al., 2019). Also, Wu et al. (2011) proposed that pre-emulsified vegetable oil may enhance the gel-forming ability and structure, depending on the oil type. A reduction in cohesiveness and adhesiveness was noted as emulgel concentration increased, indicating the disruption of the continuous protein matrix by incorporating the emulgel into the fish nugget structure. This outcome aligned with the findings of Baer and Dilger (2014), who reported that the addition of emulsified corn oil to sausage resulted in a reduction in its hardness, chewiness, and springiness. Likewise, burger patties prepared with emulsified avocado oil as a substitute for back fat exhibited reduced hardness, gumminess, and chewiness compared to those made with back fat (Rodríguez-Carpena et al., 2012). No changes in cohesiveness and adhesiveness were observed as the storage time progressed (Oppong et al., 2022). This aligns with the findings of Álvarez et al. (2012), who noted that incorporating walnut extract into a rice bran oil emulsion enhanced gel network formation and elasticity compared to back fat emulsions, with no significant changes in springiness during frozen storage. Based on the results, the inclusion of sunflower oleogel and emulgels in burgers reduced hardness and cohesiveness while increasing springiness and chewiness. Similar results for hardness and chewiness for burger were reported by Moghtadaei et al. (2018).
Laredo et al. (2011) suggested that the hardness of fats is influenced by the degree of unsaturation in fatty acids. A higher number of double bonds in triglycerides increases their flexibility, allowing for tighter aggregation. This observation aligns with our findings on the hardness of the burger samples.
3.3.3 Sensory evaluation of prepared burger
The sensory evaluation of burger samples provided insight into the effects of replacing animal fat with oleogel, emulgel, and emulgel enriched with onion phenol extract (Tab. 6). The control sample (animal fat) generally scored the highest for color (9.0), which is likely due to the natural appearance of animal fat, contributing to a familiar and appealing color. However, the burgers with oleogel and emulgel showed slightly lower color scores, indicating that the fat alternatives may alter the visual perception of the burger. The emulgel with phenolic extract performed similarly to the control, suggesting the phenolic extract may help enhance the appearance.
In terms of odor, all samples performed well, but the emulgel with onion phenol extract scored the highest (9.0), possibly due to the pleasant aromatic qualities of the extract, which may have enhanced the overall sensory profile. The flavor scores were consistent across all samples. However, the emulgel burger with onion phenol extract showed a significant difference in flavor compared to the others.
Juiciness was another important factor, where the emulgel-based burgers outperformed the control, especially the emulgel with onion phenol extract (9.0). The emulgel structure likely contributed to better water retention, enhancing the perception of juiciness, which aligns with other studies indicating that emulgels improve moisture retention in meat products. Also, the firmness showed higher score in the burgers prepared by oleogel and emulgels in comparison with control.
Overall, the sensory evaluation suggests that the inclusion of emulgel, particularly with onion phenol extract, improved the juiciness, flovor and odor without drastically compromising other attributes. This supports the potential use of oleogel and emulgel as healthier fat alternatives in meat products, which is in line with studies that report similar outcomes when replacing animal fats with structured fat alternatives.
Sensory evaluation of different burger samples.
3.4 Storage of burger samples for 14 days 4 °C
3.4.1 Weight loss (%) of stored burger samples
The weight loss observed in the burger samples stored at 4 °C for 14 days can be attributed to moisture evaporation, fat oxidation, and microbial activity, which are typical in refrigerated meat products. The control burger exhibited the highest weight loss (2.51% at Day 14), indicating that natural dehydration occurred over time (Tab.7). This finding is consistent with previous studies, which report significant moisture loss in meat products during storage, leading to changes in texture and juiciness (Kim et al., 2006). This suggested that the control burger, lacking stabilizing agents, underwent typical degradation processes such as water evaporation and oxidation.
In contrast, the oleogel burger showed reduced weight loss (2.03% at Day 14), suggesting that the oleogel structure helped retain moisture better than the control. Oleogels, which provide a stable matrix for fat retention, are known for their ability to enhance moisture retention in food products. This result is supported by earlier studies on oleogels, which showed improved water retention in various food matrices, reducing dehydration and potentially extending shelf life (Tan et al., 2023). Similarly, the emulgel burger also exhibited a decrease in weight loss (1.54 and 1.45% without and with phenolic extract, respectively at Day 14), which can be attributed to the emulgel’s water-holding capacity. Emulgels, being a combination of emulsions and gels, are designed to stabilize moisture and enhance texture, which likely helped minimize evaporation during storage (Milutinov et al., 2023).
This aligns with studies demonstrating that plant-derived phenols improve the shelf life of meat products by protecting against oxidative degradation (Ribeiro et al., 2019). Overall, these findings suggest that combining emulgels with natural antioxidants, such as phenolic compounds, offers a promising approach to improving moisture retention and stability in refrigerated food products.
Weight loss (%), firmness, pH, peroxide value, and TBARS of stored burger samples at 4 °C for 14 Days.
3.4.2 Firmness of stored burger samples
The firmness values of the burger samples, measured at Day 0, Day 7, and Day 14, provide insight into the texture changes that occurred during refrigerated storage (Tab.7). Firmness is a key indicator of the product’s textural integrity and can reflect changes in moisture content, protein denaturation, or structural degradation. The control burger showed a marked decrease in firmness over time, from 13.24 N at Day 0 to 6.80 N at Day 14. This suggests that, as the product aged, it lost moisture, resulting in a softer and less firm texture. Moisture loss, which is common in refrigerated meat products, can cause proteins to weaken, leading to reduced texture strength (Bao and Ertbjerg, 2019). Similar textural softening has been reported in various meat products during storage, with a significant reduction in firmness attributed to the loss of water and the breakdown of muscle fibers (Warner, 2023).
The oleogel burger exhibited a more moderate decline in firmness, from 13.13 N at Day 0 to 7.23 N at Day 14. Oleogels, known for their ability to retain moisture and improve texture, likely helped slow down the softening process compared to the control burger. This result aligns with research by Martins et al. (2018), which demonstrated that oleogels can enhance the structural stability and texture of fat-based products by reducing water loss and maintaining a stable fat matrix. In comparison, the emulgel burger showed a smaller decrease in firmness (from 13.57 N to 8.14 N), suggesting that the emulgel’s gel structure, which combines water retention with emulsion stability, played a role in preserving the texture of the burger. This finding supports previous studies where emulgels were shown to enhance the firmness and stability of food products, including meat analogs and fat-based foods (Martins et al., 2018).
The emulgel burger with added phenolic onion extract exhibited the least change in firmness, from 13.30 N to 8.57 N, indicating that the combination of emulgel and phenolic compounds further stabilized the texture. This is consistent with studies highlighting the role of antioxidants in maintaining the texture and quality of meat products during storage (Bellucci et al., 2022).
3.4.3 pH of stored burger samples
The pH of meat-based products is an important indicator of their chemical and microbiological stability during storage. A decrease in pH can be a sign of microbial growth, enzymatic activity, or the production of acidic by-products, which could indicate spoilage. In this study, the pH values of the burger samples decreased over time, but the rate of decrease varied between formulations. The control burger showed a gradual decline in pH, from 5.70 at Day 0 to 5.20 at Day 14, which is consistent with typical spoilage processes in meat products. As meat products age, the accumulation of lactic acid from microbial activity or enzymatic processes can lower the pH (Wang et al., 2022), and this result suggests that such mechanisms may have been at play in the control burgers.
The oleogel burger exhibited a similar trend in pH decrease (from 5.75 at Day 0 to 5.30 at Day 14), although the decline was slightly less pronounced than the control. Oleogels, by incorporating structured oils, may contribute to a more stable fat matrix, which can help reduce microbial activity and slow down pH changes. This could be attributed to the water-retention properties of oleogels, which may reduce moisture availability for microbial growth (Guo et al., 2023). The slower pH decrease in the oleogel burger may indicate a reduced rate of microbial growth or a more stable product environment compared to the control.
The emulgel burger showed a slightly higher pH at Day 14 (5.40) compared to the control and oleogel burgers, with a smaller decrease from its initial value (5.65 to 5.55 at Day 7). Emulgels, which consist of gelled emulsions, can provide a more stable water and fat distribution, potentially reducing the activity of microorganisms that contribute to pH reduction (Lu et al., 2019). Furthermore, the emulgel burger with phenolic onion extract demonstrated the smallest decline in pH, from 5.70 to 5.50, suggesting that the addition of phenolic compounds might further stabilize the product. Phenolic compounds have well-known antioxidant properties, which can help inhibit the growth of spoilage microorganisms and reduce the production of acids (Martillanes et al., 2017). These results support previous research that shows the beneficial effects of antioxidants in preserving the pH stability and shelf life of meat products (Papuc et al., 2017).
3.4.4 Oxidative stability of stored burger samples
The peroxide value (PV) is a critical indicator of lipid oxidation, which can impact the quality, safety, and shelf life of food products. A higher peroxide value indicates greater oxidation of lipids, which can lead to off-flavors, decreased nutritional quality, and compromised safety. In this study, the peroxide values of the burger samples increased over the 14-day storage period at 4 °C, reflecting the gradual oxidation of lipids in the samples.
The control burger exhibited the highest increase in peroxide value, rising from 0.12 meq O₂/kg at Day 0 to 2.20 meq O₂/kg at Day (Tab.7). This sharp increase in peroxide value suggests that the control sample was more susceptible to lipid oxidation during storage. This is consistent with previous studies that report a rapid increase in peroxide values in meat products stored under refrigeration, where exposure to oxygen and the absence of antioxidants accelerate oxidative processes (Domínguez et al., 2019). The increase in peroxide values in the control sample could be attributed to the exposure of unsaturated fatty acids to oxygen, which leads to the formation of peroxides and other oxidative products.
In contrast, the oleogel burger showed a slower increase in peroxide value, from 0.13 meq O₂/kg at Day 0 to 1.80 meq O₂/kg at Day 14. Oleogels are known to improve the stability of lipid systems by providing a structured fat matrix that can help reduce the rate of oxidation. This finding supports previous research by Hu et al. (2023), which demonstrated that oleogels can act as a barrier to oxygen diffusion, thereby slowing down lipid oxidation in fat-based products. Similarly, the emulgel burger exhibited a lower increase in peroxide value, rising from 0.11 meq O₂/kg to 1.50 meq O₂/kg. Emulgels, with their combination of emulsified fat and gel network, have been shown to reduce oxidation by stabilizing the fat and providing protection from oxidative agents (Tanislav et al., 2024).
The emulgel burger with phenolic onion extract showed the lowest increase in peroxide value, from 0.10 meq O₂/kg to 1.20 meq O₂/kg. This indicates that the addition of phenolic compounds from onion extract played a significant role in inhibiting lipid oxidation. Phenolic compounds are well-known for their antioxidant properties, which help in scavenging free radicals and preventing the formation of peroxides during lipid oxidation (Zamora and Hidalgo, 2016). Oppong et al. (2022) found that, no significant differences in PV formation were observed at any time point between the 3 g/100 g and 5 g/100 g emulgel-formulated samples, and both values were lower than those of the control group. Additionally, the reduction in PV in the EMEX burger could be attributed to the incorporation of phenolic compounds, which likely resulted in a denser internal network structure. (Hidalgo and Zamora, 2003). Increasing PV in all samples during storage was demonstrated by Sadeghinejad et al. (2019) who noticed that PV increased during the ripening period such that the highest value in oleogel with BHA and normal oleogel occurred on day 28 and in oleogel with phenolic compounds from Pistachio green hull extract on day 30.
3.4.5 TBARS of stored burger samples
The thiobarbituric acid-reactive substances (TBARS) assay is a common method for assessing lipid oxidation in food products, particularly the formation of malondialdehyde (MDA), a secondary product of lipid peroxidation. An increase in TBARS values over time indicates that lipid oxidation is occurring, which can negatively affect the flavor, nutritional quality, and safety of meat products. In this study, the TBARS values of the burger samples increased during the 14-day storage period at 4 °C, but the rate of increase varied between the different formulations.
The control burger exhibited the most significant increase in TBARS, from 0.12 mg MDA/kg at Day 0 to 1.52 mg MDA/kg at Day 14 (Tab.7). This substantial rise in TBARS values suggests that the control burger underwent considerable lipid oxidation, likely due to the exposure of unsaturated fatty acids to oxygen over time. This finding is consistent with previous studies, which have demonstrated a rapid increase in TBARS values in meat products during refrigerated storage, primarily attributed to oxidative degradation of lipids (Said dos Santos et al., 2021). Without any antioxidant protection or stabilization, the control burger is highly susceptible to lipid oxidation.
The oleogel burger showed a lower increase in TBARS, from 0.13 mg MDA/kg to 1.11 mg MDA/kg, indicating a slower rate of lipid oxidation compared to the control. Oleogels, with their structured fat matrix, have been reported to reduce the availability of free fatty acids for oxidation by trapping lipids in a gel network (Temkov and Mureșan, 2021). This stabilization of the fat matrix may limit oxygen diffusion, thereby slowing down the oxidation process. Similarly, the emulgel burger exhibited an even lower increase in TBARS values, rising from 0.10 mg MDA/kg at Day 0 to 0.93 mg MDA/kg at Day 14. The emulgel burger with phenolic onion extract showed the lowest increase in TBARS values, from 0.09 mg MDA/kg to 0.60 mg MDA/kg. The presence of phenolic compounds from onion extract significantly reduced lipid oxidation, likely due to their antioxidant properties. This result is supported by studies indicating that plant-derived phenols, such as those found in onions, can effectively reduce TBARS values and extend the shelf life of meat products by inhibiting lipid peroxidation (Gray et al., 1996).
4 Conclusion
The incorporation of onion peel phenolic extract into sunflower oil-beeswax emulgels (EMEX) provided a promising fat-replacement strategy in food applications, improving both physical and chemical properties. The enriched phenolic profile of EMEX enhanced antioxidant activity and significantly reduced lipid oxidation during storage, with lower peroxide values (4.83 meq O₂/kg) and TBARS levels (0.38 mg MDA/kg) compared to control samples. EMEX also improved the sensory qualities of burger samples, particularly in terms of appearance (8.35) and juiciness (9.0), while maintaining structural integrity with the lowest weight loss (1.08%) during frozen storage. These findings suggest that oleogels and emulgels enriched with bioactive compounds from agricultural waste, such as onion peel phenolics, could serve as a sustainable alternative to traditional animal fats in processed food products, offering both health and environmental benefits.
Acknowledgments
The authors acknowledge princess Nourah bint Abdulrahman University Researches Supporting Project number (PNURSP2025R894), princess Nourah bint Abdulraman University, Riyadh, Saudi Arabia.
Conflicts of interest
The authors declare no conflicts of interest in regards to this article.
Author contribution statement
Huda Ahmad Alamer: Writing − Original Draft, Writing − Review & Editing, Supervision, Funding acquisition
Reham M. Kamel: Writing − Original Draft, Project administration
Samar M. S. Shawir: Writing − Original Draft
Mohamed Reda Badr and Ekraam H. Barakat: Conceptualization, Writing − Review & Editing
Mohamed Abdelbaset Salama: Conceptualization, Methodology, Formal analysis, Investigation, Visualization, Writing − Original Draft, Writing − Review & Editing
References
- Abdallah DJ, Sirchio SA, Weiss RG. 2000. Hexatriacontane organogels. The first determination of the conformation and molecular packing of a low-molecular-mass organogelator in its gelled state. Langmuir 16: 7558–7561. [Google Scholar]
- Abdin M, Saleh MN, Sakr H, El-Bana M, Kamel RM, El-kholy MM, El Fadly E, Salama MA. 2024. Production of novel bio-transfer films composed from polyvinyl alcohol/sodium caseinate enhanced with bonded anthocyanins from poinsettia for minced meat preservation in double sheet system. J Food Measur Character 18: 6956–6972. [Google Scholar]
- Alshehri AA, Kamel RM, Gamal H, Sakr H, Saleh MN, El-Bana M, El-Dreny E-SG, El Fadly E, Abdin M, Salama MA. 2024. Sodium alginate films incorporated with Lepidium sativum (Garden cress) extract as a novel method to enhancement the oxidative stability of edible oil. Int J BiologMacromol 265: 130949. [Google Scholar]
- Alshehri AA, Kamel RM, Salama MA, Abdin M, Salama YA, Elsayed M. 2025. Oleogel-based fat structuring: functional, oxidative, and thermal stability of Moringa seed, Tigernut, and garden cress oils with various waxes. Int J Food Sci Technol: vvaf080. [Google Scholar]
- Álvarez D, Xiong YL, Castillo M, Payne FA, Garrido MD. 2012. Textural and viscoelastic properties of pork frankfurters containing canola-olive oils, rice bran, and walnut. Meat Sci 92: 8–15. [Google Scholar]
- Asyrul-Izhar AB, Bakar J, Sazili AQ, Meng GY, Ismail-Fitry MR. 2023. Incorporation of different physical forms of fat replacers in the production of low-fat/reduced-fat meat products: which is more practical? Food Rev Int 39: 6387–6419. [Google Scholar]
- Attard E 2013. A rapid microtitre plate Folin-Ciocalteu method for the assessment of polyphenols. Open Life Sci 8: 48–53. [Google Scholar]
- Baer AA, Dilger AC. 2014. Effect of fat quality on sausage processing, texture, and sensory characteristics. Meat Sci 96: 1242–1249. [Google Scholar]
- Bao Y, Ertbjerg P. 2019. Effects of protein oxidation on the texture and water-holding of meat: a review. Crit Rev Food Sci Nutr 59: 3564–3578. [CrossRef] [PubMed] [Google Scholar]
- Bellucci ERB, Bis-Souza CV, Domínguez R, Bermúdez R, da Silva Barretto AC. 2022. Addition of natural extracts with antioxidant function to preserve the quality of meat products. Biomolecules 12: 1506. [CrossRef] [PubMed] [Google Scholar]
- Benito-Román Ó, Blanco B, Sanz MT, Beltrán S. 2020. Subcritical water extraction of phenolic compounds from onion skin wastes (Allium cepa cv. Horcal): effect of temperature and solvent properties. Antioxidants 9: 1233. [CrossRef] [PubMed] [Google Scholar]
- Bhandari SD, Delmonte P, Honigfort M, Yan W, Dionisi F, Fleith M, Iassonova D, Bergeson LL. 2020. Regulatory changes affecting the production and use of fats and oils: Focus on partially hydrogenated oils. J Am Oil Chem Soc 97: 797–815. [Google Scholar]
- Boly R, Lamkami T, Lompo M, Dubois J, Guissou I. 2016. DPPH free radical scavenging activity of two extracts from Agelanthus dodoneifolius (Loranthaceae) leaves. Int J Toxicolog Pharmacolog Res 8: 29–34. [Google Scholar]
- Boostani S, Sarabandi K, Tarhan O, Rezaei A, Assadpour E, Rostamabadi H, Falsafi SR, Tan C, Zhang F, Jafari SM. 2024. Multiple pickering emulsions stabilized by food-grade particles as innovative delivery systems for bioactive compounds. Adv Colloid Interface Sci 103174. [Google Scholar]
- Carpintero-Tepole V, Brito-de la Fuente E, Torrestiana-Sánchez B. 2017. Microfiltration of oil in water (O/W) emulsions: effect of membrane microstructure and surface properties. Chem Eng Res Des 126: 286–296. [CrossRef] [Google Scholar]
- Celano R, Docimo T, Piccinelli AL, Gazzerro P, Tucci M, Di Sanzo R, Carabetta S, Campone L, Russo M, Rastrelli L. 2021. Onion peel: turning a food waste into a resource. Antioxidants 10: 304. [CrossRef] [PubMed] [Google Scholar]
- Chaaban H, Ioannou I, Chebil L, Slimane M, Gérardin C, Paris C, Charbonnel C, Chekir L, Ghoul M. 2017. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J Food Process Preserv 41: e13203. [Google Scholar]
- Chaijan M, Cheong L-Z., Panpipat W. 2021. Rice bran oil emulgel as a pork back fat alternate for semi-dried fish sausage. PloS One 16: e0250512. [Google Scholar]
- da Silva SL, Amaral JT, Ribeiro M, Sebastião EE, Vargas C, de Lima Franzen F, Schneider G, Lorenzo JM, Martins Fries LL, Cichoski AJ.. 2019. Fat replacement by oleogel rich in oleic acid and its impact on the technological, nutritional, oxidative, and sensory properties of Bologna-type sausages. Meat Sci 149: 141–148. [Google Scholar]
- de Souza Paglarini C, de Figueiredo Furtado G, Biachi JP, Vidal VAS, Martini S, Forte MBS, Cunha RL, Pollonio MAR. 2018. Functional emulsion gels with potential application in meat products. J Food Eng 222: 29–37. [Google Scholar]
- Doan CD, Patel AR, Tavernier I, De Clercq N, Van Raemdonck K, Van de Walle D, Delbaere C, Dewettinck K. 2016. The feasibility of wax‐based oleogel as a potential co‐structurant with palm oil in low‐saturated fat confectionery fillings. Eur J Lipid Sci Technol 118: 1903–1914. [CrossRef] [Google Scholar]
- Domínguez R, Pateiro M, Gagaoua M, Barba FJ, Zhang W, Lorenzo JM. 2019. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 8: 429. [CrossRef] [Google Scholar]
- Erdogdu SB, Erdogdu F, Ekiz HI. 2007. Influence of sodium tripolyphosphate (STP) treatment and cooking time on cook losses and textural properties of red meats. J Food Process Eng 30: 685–700. [Google Scholar]
- Ferdaus MJ, Barman B, Mahmud N, da Silva RC. 2024. Oleogels as a promising alternative to animal fat in saturated fat-reduced meat products: a review. Gels 10: 92. [Google Scholar]
- Gómez-Estaca J, Herrero AM, Herranz B, Álvarez MD, Jiménez-Colmenero F, Cofrades S. 2019. Characterization of ethyl cellulose and beeswax oleogels and their suitability as fat replacers in healthier lipid pâtés development. Food Hydrocoll 87: 960–969. [Google Scholar]
- Gray JI, Gomaa EA, Buckley DJ. 1996. Oxidative quality and shelf life of meats. Meat Sci 43: 111–123. [Google Scholar]
- Guo J, Cui L, Meng Z. 2023. Oleogels/emulsion gels as novel saturated fat replacers in meat products: a review. Food Hydrocoll 137: 108313. [Google Scholar]
- Han L, Li L, Li B, Zhao L, Liu G-q, Liu X, Wang X. 2014. Structure and physical properties of organogels developed by sitosterol and lecithin with sunflower oil. J Am Oil Chem Soc 91: 1783–1792. [Google Scholar]
- Hayes JE, Desmond EM, Troy DJ, Buckley DJ, Mehra R. 2005. The effect of whey protein-enriched fractions on the physical and sensory properties of frankfurters. Meat Sci 71: 238–243. [Google Scholar]
- Heck RT, Saldaña E, Lorenzo JM, Correa LP, Fagundes MB, Cichoski AJ, de Menezes CR, Wagner R, Campagnol PCB. 2019. Hydrogelled emulsion from chia and linseed oils: a promising strategy to produce low-fat burgers with a healthier lipid profile. Meat Sci 156: 174–182. [Google Scholar]
- Holman BWB, Coombs CEO, Morris S, Kerr MJ, Hopkins DL. 2017. Effect of long term chilled (up to 5 weeks) then frozen (up to 12 months) storage at two different sub-zero holding temperatures on beef: 1. Meat quality and microbial loads. Meat Sci 133: 133–142. [Google Scholar]
- Hu X, Jiang Q, Du L, Meng Z. 2023. Edible polysaccharide-based oleogels and novel emulsion gels as fat analogues: a review. Carbohydr Polym 121328. [Google Scholar]
- Hwang H-S 2020. A critical review on structures, health effects, oxidative stability, and sensory properties of oleogels. Biocatal Agric Biotechnol 26: 101657. [CrossRef] [Google Scholar]
- Hyatt JR, Zhang S, Akoh CC. 2023. Characterization and comparison of oleogels and emulgels prepared from Schizochytrium algal oil using monolaurin and MAG/DAG as gelators. J Am Oil Chem Soc 100: 945–959. [Google Scholar]
- Issara U, Suwannakam M, Park S. 2022. Effect of traditional fat replacement by oleogel made of beeswax and canola oil on processed meat (steak type) quality. Food Res 6: 289–299. [Google Scholar]
- Jia B, Wang W, Yoon S-C., Zhuang H, Li Y-F. 2018. Using a combination of spectral and textural data to measure water-holding capacity in fresh chicken breast fillets. Appl Sci 8: 343. [CrossRef] [Google Scholar]
- Jimenez-Colmenero F, Salcedo-Sandoval L, Bou R, Cofrades S, Herrero AM, Ruiz-Capillas C. 2015. Novel applications of oil-structuring methods as a strategy to improve the fat content of meat products. Trends Food Sci Technol 44: 177–188. [Google Scholar]
- Keskin Uslu E, Yılmaz E. 2021. Preparation and characterization of oleogels and emulgels with glycerol monooleate-cholesterol mixtures. Chem Papers 75: 2075–2085. [CrossRef] [Google Scholar]
- Kim KM, Ko JA, Lee JS, Park HJ, Hanna MA. 2006. Effect of modified atmosphere packaging on the shelf-life of coated, whole and sliced mushrooms. LWT-Food Sci Technol 39: 365–372. [Google Scholar]
- Kumar M, Barbhai MD, Hasan M, Punia S, Dhumal S, Rais N, Chandran D, Pandiselvam R, Kothakota A, Tomar M. 2022. Onion (Allium cepa L.) peels: a review on bioactive compounds and biomedical activities. Biomed Pharmacother 146: 112498. [CrossRef] [PubMed] [Google Scholar]
- Kupiec M, Zbikowska A, Marciniak-Lukasiak K, Kowalska M. 2020. Rapeseed oil in new application: assessment of structure of oleogels based on their physicochemical properties and microscopic observations. Agriculture 10: 211. [CrossRef] [Google Scholar]
- Laredo T, Barbut S, Marangoni AG. 2011. Molecular interactions of polymer oleogelation. Soft Matter 7: 2734–2743. [Google Scholar]
- Lim J, Hwang H-S., Lee S. 2017. Oil-structuring characterization of natural waxes in canola oil oleogels: rheological, thermal, and oxidative properties. Appl Biol Chem 60: 17–22. [CrossRef] [Google Scholar]
- Lu Y, Mao L, Hou Z, Miao S, Gao Y. 2019. Development of emulsion gels for the delivery of functional food ingredients: From structure to functionality. Food Eng Rev 11: 245–258. [Google Scholar]
- Manzoor S, Masoodi FA, Rashid R, Naqash F, Ahmad M. 2022. Oleogels for the development of healthy meat products: a review. Appl Food Res 2: 100212. [CrossRef] [Google Scholar]
- Marangoni AG, Garti N. 2018. Edible oleogels: structure and health implications (Elsevier). [Google Scholar]
- Maraschiello C, Sárraga C, García Regueiro F JA. 1999. Glutathione peroxidase activity, TBARS, and α-tocopherol in meat from chickens fed different diets. J Agric Food Chem 47: 867–872. [Google Scholar]
- Martillanes S, Rocha-Pimienta J, Cabrera-Bañegil M, Martín-Vertedor D, Delgado-Adámez J. 2017. Application of phenolic compounds for food preservation: food additive and active packaging. Phenolic Compounds-Biolog Activ 3: 39–58. [Google Scholar]
- Martini S, Tan CY, Jana S. 2015. Physical characterization of wax/oil crystalline networks. J Food Sci 80: C989– C97. [Google Scholar]
- Martins AJ, Cerqueira MA, Cunha RL, Vicente AA. 2017. Fortified beeswax oleogels: Effect of β-carotene on the gel structure and oxidative stability. Food Funct 8: 4241–4250. [Google Scholar]
- Martins AJ, Vicente AA, Cunha RL, Cerqueira MA. 2018. Edible oleogels: an opportunity for fat replacement in foods. Food Funct 9: 758–773. [Google Scholar]
- Martins AJ, Vicente AA, Pastrana LM, Cerqueira MA. 2020. Oleogels for development of health-promoting food products. Food Sci Human Wellness 9: 31–39. [Google Scholar]
- Meilgaard MC, Thomas Carr B, Civille GV. 1999. Sensory evaluation techniques (CRC Press). [Google Scholar]
- Mejia SMV, de Francisco A, Bohrer BM. 2019. Replacing starch in beef emulsion models with β-glucan, microcrystalline cellulose, or a combination of β-glucan and microcrystalline cellulose. Meat Sci 153: 58–65. [Google Scholar]
- Milutinov J, Krstonošić V, Ćirin D, Pavlović N. 2023. Emulgels: promising carrier systems for food ingredients and drugs. Polymers 15: 2302. [Google Scholar]
- Min C, Zhang C, Cao Y, Li H, Pu H, Huang J, Xiong YL. 2023. Rheological, textural, and water-immobilizing properties of mung bean starch and flaxseed protein composite gels as potential dysphagia food: the effect of Astragalus polysaccharide. Int J BiologMacromol 239: 124236. [Google Scholar]
- Moghtadaei M, Soltanizadeh N, Goli SAH. 2018. Production of sesame oil oleogels based on beeswax and application as partial substitutes of animal fat in beef burger. Food Res Int 108: 368–377. [Google Scholar]
- Naderizadeh S, Heredia‐Guerrero JA, Caputo G, Grasselli S, Malchiodi A, Athanassiou A, Bayer IS. 2019. Superhydrophobic coatings from Beeswax‐in‐water emulsions with latent heat storage capability. Adv Mater Interfaces 6: 1801782. [CrossRef] [Google Scholar]
- Ngo TV, Kusumawardani S, Kunyanee K, Luangsakul N. 2022. Polyphenol-modified starches and their applications in the food industry: recent updates and future directions. Foods 11: 3384. [Google Scholar]
- O’Sullivan CM, Barbut S, Marangoni AG. 2016. Edible oleogels for the oral delivery of lipid soluble molecules: Composition and structural design considerations. Trends Food Sci Technol 57: 59–73. [Google Scholar]
- Öğütcü M, Yılmaz E. 2015. Characterization of hazelnut oil oleogels prepared with sunflower and carnauba waxes. Int J Food Properties 18: 1741–1755. [Google Scholar]
- Okuro PK, Tavernier I, Bin Sintang MD, Skirtach AG, Vicente AA, Dewettinck K, Cunha RL. 2018. Synergistic interactions between lecithin and fruit wax in oleogel formation. Food Funct 9: 1755–1767. [Google Scholar]
- Oppong D, Panpipat W, Cheong L-Z., Chaijan M. 2022. Rice flour-emulgel as a bifunctional ingredient, stabiliser-cryoprotactant, for formulation of healthier frozen fish nugget. LWT 159: 113241. [Google Scholar]
- Paesa M, Nogueira DP, Velderrain-Rodríguez G, Esparza I, Jiménez-Moreno N, Mendoza G, Osada J, Martin-Belloso O, Rodríguez-Yoldi MJ, Ancín-Azpilicueta C. 2022. Valorization of onion waste by obtaining extracts rich in phenolic compounds and feasibility of its therapeutic use on colon cancer. Antioxidants 11: 733. [CrossRef] [PubMed] [Google Scholar]
- Papuc C, Goran GV, Predescu CN, Nicorescu V, Stefan G. 2017. Plant polyphenols as antioxidant and antibacterial agents for shelf‐life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Comprehen Rev Food Sci Food Saf 16: 1243–1268. [CrossRef] [Google Scholar]
- Patel AR 2015. Alternative routes to oil structuring (Springer). [Google Scholar]
- Patel AR, Cludts N, Bin Sintang MD, Lewille B, Lesaffer A, Dewettinck K. 2014. Polysaccharide‐based oleogels prepared with an emulsion‐templated approach. ChemPhysChem 15: 3435–3439. [CrossRef] [PubMed] [Google Scholar]
- Pintado T, Cofrades S. 2020. Quality characteristics of healthy dry fermented sausages formulated with a mixture of olive and chia oil structured in oleogel or emulsion gel as animal fat replacer. Foods 9: 830. [Google Scholar]
- Pinto TC, Martins AJ, Pastrana L, Pereira MC, Cerqueira MA. 2021. Oleogel-based systems for the delivery of bioactive compounds in foods. Gels 7: 86. [Google Scholar]
- Poti JM, Braga B, Qin B. 2017. Ultra-processed food intake and obesity: what really matters for health—processing or nutrient content?. Curr Obes Rep 6: 420–431. [CrossRef] [PubMed] [Google Scholar]
- Pușcaș A, Mureșan V, Socaciu C, Muste S. 2020. Oleogels in food: a review of current and potential applications. Foods 9. [Google Scholar]
- Remig V, Franklin B, Margolis S, Kostas G, Nece T, Street JC. 2010. Trans fats in America: a review of their use, consumption, health implications, and regulation. J Am Dietetic Assoc 110: 585–592. [Google Scholar]
- Ribeiro JS, Cordeiro Santos MJM, Silva LKR, Pereira LCL, Santos IA, da Silva Lannes SC, da Silva MV. 2019. Natural antioxidants used in meat products: a brief review. Meat Sci 148: 181–188. [Google Scholar]
- Rodríguez-Carpena JG, Morcuende D, Estévez M. 2012. Avocado, sunflower and olive oils as replacers of pork back-fat in burger patties: effect on lipid composition, oxidative stability and quality traits. Meat Sci 90: 106–115. [CrossRef] [PubMed] [Google Scholar]
- Sadeghinejad N, Sarteshnizi RA, Gavlighi HA, Barzegar M. 2019. Pistachio green hull extract as a natural antioxidant in beef patties: effect on lipid and protein oxidation, color deterioration, and microbial stability during chilled storage. LWT 102: 393–402. [Google Scholar]
- Sahu S, Ghosh M, Bhattacharyya DK. 2020. Utilization of unsaponifiable matter from rice bran oil fatty acid distillate for preparing an antioxidant-rich oleogel and evaluation of its properties. Grasas Y Aceites 71: a336–a36. [Google Scholar]
- Said dos Santos R, da Silva JB, Rosseto HC, Vecchi CF, da Silva Souza Campanholi K, Caetano W, Bruschi ML. 2021. Emulgels containing propolis and curcumin: the effect of type of vegetable oil, poly (acrylic acid) and bioactive agent on physicochemical stability, mechanical and rheological properties. Gels 7: 120. [Google Scholar]
- Salama MA, Abdin M, Saleh MN, El-Baset A, Walid S, Hendawy E, Ibrahim A. 2024. Preparation and evaluation of oleogels incorporated with Moringa oleifera leaves extract in biscuits production. Food Technol Res J 4: 1–15. [Google Scholar]
- Salama MA, Harkaoui SE, Nounah I, Sakr H, Abdin M, Owon M, Osman M, Ibrahim A, Charrouf Z, Matthäus B. 2020. Oxidative stability of Opuntia ficus-indica seeds oil blending with Moringa oleifera seeds oil. OCL 27: 53. [Google Scholar]
- Sato ACK, Moraes KEFP, Cunha RL. 2014. Development of gelled emulsions with improved oxidative and pH stability. Food Hydrocoll 34: 184–192. [Google Scholar]
- Silva PM, Cerqueira MA, Martins AJ, Fasolin LH, Cunha RL, Vicente AA. 2022. Oleogels and bigels as alternatives to saturated fats: A review on their application by the food industry. J Am Oil Chem Soc 99: 911–923. [Google Scholar]
- Singh P, Wani AA, Saengerlaub S, Langowski H-C. 2011. Understanding critical factors for the quality and shelf-life of MAP fresh meat: a review. Crit Rev Food Sci Nutr 51: 146–177. [CrossRef] [PubMed] [Google Scholar]
- Sivakanthan S 2024. Development and Characterization of Rice Bran Oil and Sesame Oil-Based Oleogels for Healthy Food Applications. Queensland University of Technology. [Google Scholar]
- Sivakanthan S, Fawzia S, Mundree S, Madhujith T, Karim A. 2023. Optimization and characterization of new oleogels developed based on sesame oil and rice bran oil. Food Hydrocoll 142: 108839. [Google Scholar]
- Souad B 2024. Hydrogenated oils and public health: a scientific analysis of trans fats and disease. Braz J Health Rev 7: e74771–e71. [CrossRef] [Google Scholar]
- Steel CJ, Carmen Dobarganes M, Barrera-Arellano D. 2005. The influence of natural tocopherols during thermal oxidation of refined and partially hydrogenated soybean oils. Grasas Y Aceites 56: 46–52. [Google Scholar]
- Stoica F, Rațu RN, Veleșcu ID, Stănciuc N, Râpeanu G. 2023. A comprehensive review on bioactive compounds, health benefits, and potential food applications of onion (Allium cepa L.) skin waste. Trends Food Sci Technol 141: 104173. [Google Scholar]
- Sun H, Xu J, Lu X, Xu Y, Regenstein JM, Zhang Y, Wang F. 2022. Development and characterization of monoglyceride oleogels prepared with crude and refined walnut oil. LWT 154: 112769. [Google Scholar]
- Tabibazar M, Hamishekar H, Alizadeh A. 2020. Assessing the feasibility of carnauba wax/adipic acid oleogel application as a shortening replacer in cake. J Food Sci Technol 17: 83–93. [Google Scholar]
- Tan TH, Chan E‐S, Manja M, Tang T‐K, Phuah E‐T, Lee Y‐Y. 2023. Production, health implications and applications of oleogels as fat replacer in food system: a review. J Am Oil Chem Soc 100: 681–697. [Google Scholar]
- Tanislav AE, Pușcaș A, Păucean A, Mureșan AE, Semeniuc CA, Mureșan V, Mudura E. 2022. Evaluation of structural behavior in the process dynamics of oleogel-based tender dough products. Gels 8: 317. [Google Scholar]
- Tanislav AE, Pușcaș A, Mureșan V, Mudura E. 2024. The oxidative quality of bi-, oleo-and emulgels and their bioactives molecules delivery. Crit Rev Food Sci Nutr 64: 8990–9016. [CrossRef] [PubMed] [Google Scholar]
- Temkov M, Mureșan V. 2021. Tailoring the structure of lipids, oleogels and fat replacers by different approaches for solving the trans-fat issue—a review. Foods 10: 1376. [CrossRef] [PubMed] [Google Scholar]
- Tian H, Fu J, Li L, Yu H, Chen C, Ma X, Lou X. 2025. Impacts of carbohydrate-based fat substitutes on the textural, rheological, microstructural, and sensory characteristics of low-fat processed cheese. LWT: 117673. [Google Scholar]
- Tian Y, Acevedo NC. 2020. Role of supramolecular policosanol oleogels in the protection of retinyl palmitate against photodegradation. RSC Adv 10: 2526–2535. [Google Scholar]
- Wang D, Cheng F, Wang Y, Han J, Gao F, Tian J, Zhang K, Jin Y. 2022. The changes occurring in proteins during processing and storage of fermented meat products and their regulation by lactic acid bacteria. Foods 11: 2427. [Google Scholar]
- Warner RD 2023. The eating quality of meat: IV—Water holding capacity and juiciness.’ in, Lawrie’s meat science (Elsevier). [Google Scholar]
- Willett SA, Akoh CC. 2019. Physicochemical characterization of organogels prepared from menhaden oil or structured lipid with phytosterol blend or sucrose stearate/ascorbyl palmitate blend. Food Funct 10: 180–190. [Google Scholar]
- Wu M, Xiong YL, Chen J. 2011. Rheology and microstructure of myofibrillar protein-plant lipid composite gels: effect of emulsion droplet size and membrane type. J Food Eng 106: 318–324. [Google Scholar]
- Xia X, Zhao Z, Cai W, Li C, Yang F, Yao B, Sun G. 2022. Effects of paraffin wax content and test temperature on the stability of water-in-model waxy crude oil emulsions. Colloids Surf A: Physicochem Eng Aspects 652: 129815. [CrossRef] [Google Scholar]
- Yaqoob, N., Rehman, S., Shafiq, N., Mohsin, M., Akbar, A., Ibenmoussa, S., Wondmie, G.F., Bin Jardan, Y.A. and Bourhia, M., 2024. Synthesis and characterization of antioxidant-enriched Moringa oil-based edible oleogel. Open Chemistry, 22(1), p.20240038. [Google Scholar]
- Yılmaz E 2022. Sensory properties and aromatics profile of edible oleogels’. [Google Scholar]
- Yilmaz E, Demirci Ş. 2021. Preparation and evaluation of virgin olive oil oleogels including thyme and cumin spices with sunflower wax. Gels 7: 95. [Google Scholar]
- Yılmaz E, Öğütcü M. 2014. Properties and stability of hazelnut oil organogels with beeswax and monoglyceride. J Am Oil Chem Soc 91: 1007–1017. [Google Scholar]
- Yılmaz E, Öğütcü M. 2015. The texture, sensory properties and stability of cookies prepared with wax oleogels. Food Funct 6: 1194–1204. [Google Scholar]
- Youssef MK, Barbut S. 2009. Effects of protein level and fat/oil on emulsion stability, texture, microstructure and color of meat batters. Meat Sci 82: 228–233. [Google Scholar]
- Zamora R, Hidalgo FJ. 2016. The triple defensive barrier of phenolic compounds against the lipid oxidation-induced damage in food products. Trends Food Sci Technol 54: 165–174. [Google Scholar]
- Zampouni K, Soniadis A, Dimakopoulou-Papazoglou D, Moschakis T, Biliaderis CG, Katsanidis E. 2022. Modified fermented sausages with olive oil oleogel and NaCl-KCl substitution for improved nutritional quality. LWT 158: 113172. [Google Scholar]
- Zhang H 2020. Rice bran wax oleogel in an oil-in-water emulsion system. University of Nottingham. [Google Scholar]
- Zhang Q, Cheng Z, Wang Y, Fu L. 2021. Dietary protein-phenolic interactions: Characterization, biochemical-physiological consequences, and potential food applications. Crit Rev Food Sci Nutr 61: 3589–3615. [CrossRef] [PubMed] [Google Scholar]
- Zhang X, Tao N, Wang X, Chen F, Wang M. 2015. The colorants, antioxidants, and toxicants from nonenzymatic browning reactions and the impacts of dietary polyphenols on their thermal formation. Food Funct 6: 345–355. [Google Scholar]
- Zhang X, Guo Y, Liu H, Liang B, He H, Fu X, Sun C, Li X, Ji C. 2023. Preparation, characterization of curdlan-based emulsion micro-gel particles and its application in low-fat pork sausages. LWT 185: 115160. [Google Scholar]
- Zhou M, Li B, Wu A, Hu Z, Liu J, Wang Y, Liu H. 2024. Preparation of a bigel system based on k-carrageenan hydrogel and beeswax oleogel and the effect of starch on the bigel properties. LWT 205: 116516. [Google Scholar]
Cite this article as: Alamer HA, Kamel RM, Shawir SMS, Salama MA, Barakat EH. 2025. Utilizing phenolic-enriched sunflower Oleogels and Emulgels as fat replacers: impact on burger oxidative stability, texture, and sensory attributes. OCL 32: 21. https://doi.org/10.1051/ocl/2025017
All Tables
Total phenolic content (TPC), antioxidant activity (DPPH) and chemical properties of sunflower oil-beeswax oleogel and emulgel.
Weight loss (%), firmness, pH, peroxide value, and TBARS of stored burger samples at 4 °C for 14 Days.
All Figures
 |
Fig. 1 (A1) Sunflower oil, (A2) Oleogel, (A3) Emulgel, (A4) Emulgel with phenolic onion extract; (B1) Control burger, (B2) Oleogel burger, (B3) Emulgel burger, (B4) Emulgel with phenolic onion extract burger. |
| In the text | |
 |
Fig. 2 Physical, color, texture and viscosity properties of sunflower oil-beeswax oleogel and emulgel. OLEO = sunflower oleogel, EM = sunflower emulgel, EMEX = sunflower emulgel with onion peel phenolic extract. |
| In the text | |
 |
Fig. 3 AFM, SEM and DSC of sunflower oil-beeswax oleogel and emulgel. |
| In the text | |
 |
Fig. 4 Texture analysis of burger samples. OLEO = sunflower oleogel, EM = sunflower emulgel, EMEX = sunflower emulgel with onion peel phenolic extract. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.




