| Issue |
OCL
Volume 31, 2024
Lipids from aquatic environments / Lipides issus des milieux aquatiques
|
|
|---|---|---|
| Article Number | 9 | |
| Number of page(s) | 13 | |
| Section | Quality - Food safety | |
| DOI | https://doi.org/10.1051/ocl/2024005 | |
| Published online | 15 May 2024 | |
Research article
Variations in Chlorella lipid content in commercial and in-lab produced biomass☆
1
CIRAD, UMR QUALISUD, 34398 Montpellier, France
2
QUALISUD, Univ Montpellier, Avignon Université, CIRAD, Institut Agro, Université de La Réunion, Montpellier, France
3
UMR IATE, Univ Montpellier, INRAE, Institut Agro, Montpellier, France
4
UMR STLO, INRAE, Institut Agro, 35000, Rennes, France
* Corresponding author: maeva.subileau@supagro.fr
Received:
21
July
2023
Accepted:
8
February
2024
Microalgae appear as a sustainable source of biomass with relevant nutritional qualities. Still, regulatory restrictions currently limit the use of eukaryotic microalgae for human consumption to a short list of species dominated by Chlorella spp. Chlorella biomass contains valuable proteins but also interesting lipids, including polyunsaturated fatty acids (PUFA) ω3 and ω6. The amount of PUFA and the ω6/ω3 ratio vary significantly depending on the species and cultivation trophic mode. While the lipid profils of in-lab produced Chlorella has been widely studied, the variability of lipid content in commercial biomasses is barely described. Here, lipid classes and fatty acid profiles of six commercial biomasses of Chlorella spp. as well as those of lab-produced C. sorokiniana grown in photo-autotrophy and in four mixotrophy conditions were characterized. Results showed significant lipid composition variations between the biomasses, such as the triacylglycerols/glycolipids and ω6/ω3 contents. The ω6/ω3 ratios were lower in photo-autotrophic mode (2.5) while they ranged between 1.3 and 8.9 in commercial biomasses. The free fatty acids level was also variable (1.4% to 17.9% of total lipids). As a consequence, Chlorella lipid content and quality differed significantly, impacting the potential nutritional benefits of the consumption of commercial biomass. Processing and post-processing conditions should therefore be carefully controlled to optimize lipid profiles.
Résumé
Les microalgues sont une ressource durable de biomasse aux qualités nutritionnelles précieuses. Toutefois, des contraintes réglementaires limitent leur consommation humaine, privilégiant les espèces de Chlorella. Les Chlorella contiennent des protéines mais aussi des lipides d’intérêt, notamment des acides gras polyinsaturés (AGPI) ω3 et ω6 dont les teneurs varient significativement selon l’espèce et le mode trophique de culture. Bien que les profils lipidiques de Chlorella aient été largement étudiés en laboratoire, ceux des biomasses commerciales sont peu décrits. Les classes de lipides et profils d’acides gras de six biomasses commerciales de Chlorella spp. et d’une souche de C. sorokiniana produite en laboratoire, en photo-autotrophie et mixotrophies, ont été caractérisés dans cette étude. Des compositions lipidiques différentes ont été obtenues, notamment pour les ratios triacylglycérols/glycolipides et ω6/ω3. En laboratoire la biomasse cultivée en photo-autotrophie présentait le plus bas ratio ω6/ω3 (2.5), tandis qu’il fluctuait entre 1.3 et 8.9 dans les commerciales. Leur taux en acides gras libres variait également de manière importante (1.4 à 17.9 % des lipides totaux). Finalement, la teneur et la qualité lipidique des Chlorelles différaient significativement, impactant leurs potentiels bénéfices nutritionnels. Un contrôle des conditions de production et post-traitement est donc nécessaire pour optimiser les profils en lipides.
Key words: Chlorella / commercial biomass / lipids / ω6/ω3 fatty acids / photoautotrophy / mixotrophy
Mots clés : Chlorella / biomasses commerciales / lipides / acides gras ω6/ω3 / photoautotrophie / mixotrophie
© N. Barouh et al., Published by EDP Sciences, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Highlights
Biotic and abiotic parameters modify the lipid composition of Chlorella biomass.
Valuable ratios of ω6/ω3 (<4) and triacylglycerols/glycolipids can be achieved in Chlorella when produced in photo-autotrophy.
Lipid quality of in-lab cultivated Chlorella is not directly scalable to that of commercial biomass.
Lipid composition of commercial Chlorella biomasses and related nutritional benefits vary significantly.
Biotic and abiotic cultivation parameters shall be considered when evaluating the benefits of microalgae consumption.
1 Introduction
The production of microalgae for therapeutic, nutraceutical and human consumption, has emerged over the last three decades as a promising sector with great economic and environmental potentials (Araújo et al., 2021; Cuellar‐Bermudez et al., 2015; Fernández et al., 2021; Katiyar and Arora, 2020; Kiran and Venkata Mohan, 2021). Microalgae are rich sources of bioactive molecules, encompassing macronutrients such as proteins, polysaccharides, and lipids, as well as micronutrients like vitamins and pigments, rendering them highly desirable for applications in food and health (Ferreira de Oliveira and Bragotto, 2022; Wong et al., 2022). Currently, two primary modalities exist for the commercialization of microalgae: the first involves the distribution of whole microalgae in a dehydrated state, while the latter entails extracts with targeted molecules of interest (Araujo and Peteiro, 2021). Regarding the consumption of whole biomass in Europe, the legislative framework is governed by the European Community Regulation on Food Safety, promulgated in 2002 in the Official Journal of the European Communities (Directive 2002/46/CE, n.d.) This regulatory landscape is notably stringent, with the authorized products "Spirulina" and "Chlorella" dominating the market (Araújo et al., 2021). Chlorella microalgae thus emerges as the predominant eukaryote species in Europe, exhibiting the highest production volumes of dried algae, quantified at 82 tonnes of dry weight annually and involving the participation of 30 companies (Araújo et al., 2021). Taxonomically affiliated with the Chlorophyta branch and the Chlorellaceae family, Chlorella comprises a total of thirteen identified species, with Chlorella vulgaris notably standing out as the most frequently commercialized species on the market.
Chlorella is currently marketed for its protein content ranging from 11% to 58%, and its vitamin composition, including β-carotene (180 mg/100 g), Biotin (191.6 mg/100 g), vitamin B12 (125.9 mg/100 g), and vitamin E (<1 mg/100 g) (Mobin and Alam, 2017). In relation with these constituents, studies have highlighted the benefits of microalgae consumption (Kumar et al., 2022; Sherafati et al., 2022). Chlorella biomass also contains other potential molecules of interest, particularly health-promoting functional lipids such as polyunsaturated fatty acids (PUFA) ω3 and ω6, that are crucial components in the human diet (Bito et al., 2020). The recommended dietary allowance for the precursor of ω6 series (i.e. linoleic acid C18:2 ω6 (LA)) is 4% of Total Energy Intake (TEI), and of 1 % of TEI for the precursor of ω3 series (i.e. α-linolenic acid C18:3 ω3 (ALA)), with an ideal ratio ω6/ω3 of 4:1 (AFSSA, 2011; Legrand, Philippe, 2013). LA and ALA can be found in marine sources but are also abundant in higher plants leaves, and in some seeds, nuts and oils (flaxseeds, soybean oil, rapessed oil, walnuts, etc.). These PUFA play crucial roles in the prevention of non-communicable diseases and generally lack in modern Western diet (Saini and Keum, 2018; Simopoulos, 2016).
In Chlorella spp., the quantity of lipids, fatty acid profiles, and molecular classes vary depending on the species and cultivation conditions, including trophic mode (autotrophy, heterotrophy, mixotrophy), nutrient limitations, and depletions (Couto et al., 2021; White et al., 2019; Yun et al., 2021, Yun et al., 2020). Adjustment of abiotic parameters such as the carbon nutrient sources and concentrations, light, temperature and salinity has been widely studied in Chlorella cultivation. In this context, most studies on Chlorella lipids have focused on fatty acid profiles, excluding details on lipid classes and on free fatty acid levels (FFA, also referred as “non-esterified” acids), both of which are crucial descriptors of microalgae lipid quality and bioaccessibility (Kergomard et al., 2021).
In-depth lipidomic studies of Chlorella have revealed variations in lipid molecular classes, especially considering proportions of membrane lipids (e.g. glycosylglycerides such as galactolipids, phosphoglycerides, betaine lipids, ether lipids) and storage lipids (e.g. triacylglycerol (TAG)) (Couto et al., 2023; White et al., 2019). Galactolipids, specifically monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG), are major polar lipids in Chlorella’s photosynthetic membranes (Petroutsos et al., 2014). Couto et al. (2021) investigated the polar lipidome of C. vulgaris under autotrophic (C-Auto) and heterotrophic (C-Hetero) conditions, finding higher ω3 abundance in autotrophic conditions along with increased unsaturated glycolipids. White et al. (2019) highlighted that polar lipid contents and fatty acids profiles in Chlorella sp. evolve during cultivation, particularly under nitrogen-limiting conditions, favoring PUFA accumulation, especially ALA and C16:4. Other studies revealed qualitative consistency but quantitative differences in fatty acid profiles based on cultivation conditions (Petkov and Garcia, 2007; Yun et al., 2021, Yun et al., 2020). Additionally, significant differences based on extraction and quantification methods were identified, complicating comparisons across studies (e.g. sum of total FA vs total lipid weighted after extraction) (Couto et al., 2022; Jones et al., 2012).
Altogether, these studies confirm that, due to their central role in photosynthetic metabolism, galactolipids are often the primary components of the microalgae lipid fraction cultivated in photo-autotrophy. In Chlorella species, these chloroplastic galactolipids predominantly consist of ω3-PUFA, including ALA and 7,10,13-hexadecatrienoic acid (C16:3 ω3, roughanic acid (RoA)) (Couto et al., 2023; White et al., 2019).
Nowadays, a diverse array of commercial products of Chlorella strains exists, mostly under the form of dried cells (powder), encompassed in various brands, often emphasizing critical factors such as protein, chlorophyll and vitamin B12 content. Additionally, ω3 content occasionally serves as a distinctive yet non-superfluous consideration among these products. Regarding production methods, cell wall rupture is sometimes mentioned, but no information related to cultivation or harvesting procedures is provided. In contrast to well-documented characterisation of laboratory biomass, scant attention has been given in the literature to the lipid content and composition of commercial biomass. In a study by Canelli et al. (2020), the composition of four commercial Chlorella strains and one Auxenochlorella strain was examined. The biomasses demonstrated significant disparities in lipid content and qualities. Specifically in FA profiles, the range was 7% to 15.8% for ALA and 27.7% to 37.5% for LA contents (% of total fatty acids), respectively. This underscores the potential significance of lipid composition and highlights the urgent need of increasing available information about commercial biomass, and narrow the gap in knowledge between well-mastered laboratory-derived microalgae composition and commercial ones.
Due to the absence of information regarding production methods (autotrophy, mixotrophy, etc.) and a lack of detailed compositional data, this study seeks to bridge this gap by comparing five distinct commercial Chlorella brands in terms of lipid classes and fatty acid composition (Tab. 1) and compare them to well-controlled laboratory productions. Since lipid composition in the biomass strongly depends on the cultivation trophic mode, we set the hypothesis that various cultivation conditions of Chlorella sorokiniana explored in laboratory would trigger various ω6/ω3 ratio and lipid quality. These conditions encompassed both photo-autotrophy and mixotrophy, with the latter achieved by introducing varying concentrations of glucose ranging from 2 to 10 g/L. Considering ω3 as a key parameter for nutritional benefit potential, our goal in comparing the lipids of commercial and laboratory biomasses is to provide insights into understanding the origin of lipid quality variability in commercial biomasses and to suggest means for controlling and improving it.
Characteristics of Chlorella spp. commercial biomasses.
2 Materials and methods
2.1 Material
Six commercial biomasses were purchased in local supermarket in Montpellier or online. Four of them are labelled as C. vulgaris species commercialized under three different brands (named A, B, C in this study), one as C. pyrenoidosa and one as C. sorokiniana (Tab. 1).
For in-lab cultivation, a strain of C. sorokiniana “Reyto” isolated from the bog of Rey (alkaline marshes, Midi Pyrénées region) by LBE (Laboratoire de Biotechnologie de l’Environnement, Narbonne, INRAE, France) and identified as identical to C. sorokiniana SAG 211-8k by 18S, 28S and ITS sequencing, was used.
All macronutrients and minerals required to produce Bold’s Basal Medium (BBM), all analytical standards (triacylglycerol (TAG), free fatty acid, (FFA), monogalactosyldiacylglycerol (MGDG), digalactosyldiacylglycerol (DGDG), phosphatidylethanolamine (PE) Phosphatidylcholine (PC), sulfoquinovosyldiacylglycerol (SQDG), Mix 37 FAME, sodium methylate, acetyl chloride, and all usual solvents were purchased from Sigma-Aldrich (Saint Quentin Fallavier, France). 7(Z),10(Z),13(Z)-Hexadecatrienoic acid and 7(Z),10(Z)-hexadecadienoic acid methyl esters were purchased from Larodan (Solna, Sweden).
2.2 Methods
2.2.1 Medium
Cultures were performed in 100 mL Erlenmeyer flasks in Bold’s Basal Medium (BBM) (Bischoff and Bold, 1963; Bold, 1949). BBM is made of six macronutrient stock solutions, alkaline EDTA solution, acidified iron solution, boron solution and trace metals solution, all prepared in ultrapure water. All macronutrient solutions were made individually: NaNO3 (25 g/L), CaCl2.2H2O (2.50 g/L), MgSO4.7H2O (7.7 g/L), K2HPO4 (7.5 g/L), KH2PO4 (17.5 g/L), NaCl (2.5 g/L) and 10 mL of each were added to 936 mL of ultrapure water.
Remaining solutions were also made individually and 1 mL of each was added to the medium before autoclaving. Alkaline EDTA solution is made up of EDTA (50 g/L) and KOH (31 g/L). Acidified iron solution was made by hydro solubilisation of FeSO4, 7H20 (4.98 g/L) by adding 40 µL of H2SO4 9 M in 50 mL of ultrapure water. Trace metal solution is made of various components: H3BO3 (0.6 g/L), ZnSO4 · 7H2O (1.2 g/L), MnCl2 · 4H2O (2.0 g/L), CuSO4 · 5H2O (250 mg/L), CoCl2 · 6H2O (250 mg/L), NaFeEDTA (7.5 g/L), Na2-EDTA · 2H2O (15.0 g/L), and NaMoO4 · 2H2O (250 mg/L). Medium was autoclaved at 120 °C for 20 min, supplemented with vitamins (Thiamine HCl at 2.96 × 10−7 M, Biotin at 4.09 × 10−9 M and Cyanocobalamin at 1.48 × 10−9 M in final media) and inoculated.
Originally, BBM does not contain any organic carbon and is therefore suitable for photoautotrophic culture. However, it can be enriched with carbon substrate. In this study, to supplement the medium, a solution of glucose was chosen for its acceptability in the food industry. A 60 g/L glucose solution in ultrapure water was made to supplement the medium with glucose and autoclaved. Concentrated BBM medium (×2) was prepared and different volumes of ultrapure water were added according to the volume of glucose solution used, to adjust and standardize mineral and nutrient concentrations in every final media.
2.2.2 Cultivation conditions
Erlenmeyer flasks (100 mL working volume) were placed in an incubator (Innova 44/44R, New Brunswick Scientifics, NJ, USA) with LED lightening (1000–2000 lux), incubated at 25 ± 1 °C and rotation of the plate was at 110 rpm in order to limit self-shadowing. Erlenmeyer flasks were filled to 1/10th of their volume to optimize CO2 transfer. Two pre-cultivations were realized for each cultivation condition and inoculum of 1/10th of the working volume was used when the optical density (OD) of the pre-cultivation reached 2. Pre-cultivations allowed microalgae to adapt to environmental conditions with glucose concentrations and stabilize metabolism in the different trophic modes implemented.
2.2.3 Growth monitoring and cultivation time
Growth monitoring was performed measuring OD by spectrophotometry at 750 nm using an Ultrospec 2100 Pro UV-visible spectrophotometer from Biochrom (Cambridge, UK). 1 mL of culture was collected under sterile conditions and placed in the spectrophotometer cells.
To determine dry biomass weight, 5 mL of culture were placed on a 0.45 μm Ø 25 MM XILAB® cellulose acetate filter put on a Buchner funnel with glass frit under vacuum. The filter was rinsed several times with distilled water and then the filter was placed in an oven at 100 °C for desiccation during 24 h.
2.2.4 Harvest of Chlorella sorokiniana Reyto
Samples from small volumes of culture (<2 mL) were concentrated by centrifugation in an Eppendorf® Centrifuge (5427 R G, B. Braun, Melsungen, Hesse, Germany) at 4000 g during 8 min at 4 °C and then submitted to thermal processing (10 min at 100 °C in a water bath) before storage at −20 °C, to limit lipolysis before extraction.
2.2.5 Total lipids
2.2.5.1 Estimation of total lipid content by phospho-vanillin assay for in lab-cultivated biomass
For analysis of lipid content in small amount of humid matter, the method described by Johnson et al. (1977) and adapted by Mishra et al. (2014) was used as follows. Total lipids were measured in 1 mL concentrated lab-cultivated biomass, placed in 10 mL tubes. 2 mL of 98% H2SO4 was first added and tubes were manually shaken. Tubes were heated in a water bath at 100 °C for 10 min and then cooled in ice for 5 min. 5 mL of phospho-vanillin reagent (0.5 mM in water/ethanol 45:5, v/v)) was added to each sample and the tubes were instantly shaken by hand. Tubes were incubated at 40 °C for 15 min at 200 rpm (Innova 44/44R, New Brunswick Scientifics, NJ, USA), then placed at room temperature in the dark for 45 min. Samples were then transferred to spectrophotometer cells and their absorbance was read at 530 nm (Ultrospec 2100 Pro UV-visible from Biochrom, Cambridge, UK). Two standard ranges from rapeseed oil and lipid extracted from C. sorokiniana Reyto in MTBE/MeOH (10:3, v/v) were performed for each determination.
2.2.5.2 Extraction of total lipid content by Folch method for commercial biomass
In order to extract lipids, the Folch extraction procedure was applied, using chloroform/methanol/water (8:4:3, v/v/v) mixture, with a solvent to sample ratio of 20:1 (Folch et al., 1957). Briefly, 5 mL of water and 40 mL of Folch solution were added to 2 g of commercial biomass and the mixture was passed through a lab disperser (Ultra-turrax, IKA, T18 digital) for 1 min at between 9,500 and 13,500 rpm. In a 500 mL separating funnel, the solution was washed once with 22.5 mL of NaCl solution (0.73%), then twice with 50 mL of a solution containing 40 mL of Folch and 10 mL of NaCl solution (0.58%). The chloroformic phase (lower) was collected between each wash and solvents were evaporated under reduced pressure.
2.2.5.3 Extraction of total lipids by MTBE/MeOH method for in-lab biomasses
A small-volume extraction method was optimized for in-lab Chlorella cultures. Between 12 mL and 15 mL of culture were collected and centrifuged at 4000 g during 8 min at 4°C (centrifuge 5427 R, Eppendorf, Montesson, France). Supernatant was removed and the pellets were boiled 10 min at 100 °C. Samples were transferred to Wheaton vials (4 mL) and two volumes of MTBE/MeOH (10:3, v/v) solvent mixture containing BHT (0.1 mg/mL) were added, along with glass microbeads and an ogive-shaped magnet bar. The mixture was stirred up (500 RPM, at 40 °C for 17 h) on a heated multi-station stirrer (Starfish Work Station, Schwabach, Germany). Volume of solvent added should be at least 600 μL to avoid evaporation during extraction. Sample were then transferred to a 2 mL Eppendorf tube, and 100 μL of 0.8% KCl have been added. Mixture was vortexed and centrifuged at 16,000 g for 10 min at 4 °C. The organic phase was collected in vial and a second extraction was carried out on the aqueous phase under the same conditions as the first, this time for only 2 h. Samples were prepared in biological triplicates and stored at −80 °C. Beforehand, tests were conducted to compare extracts obtained by this method and by Folch extraction and demonstrated that extract contents (lipid classes, fatty acid composition) were fully comparable (data not shown).
2.2.6. Lipid classes composition
The lipid extract previously obtained were analyzed according to the following procedure: Thin Layer Chromatography (TLC) was carried out on HPTLC silica gel 60 pre-coated plates (Merck, Darmstadt, Germany). Lipid extracts obtained as previously described and standard solutions were sprayed on 3 mm width bands, using a CAMAG ATS4 apparatus (Muttenz, Switzerland).
In order to visualize all lipid compounds of interest, a two-step development was achieved on HPTLC silica gel 60 pre-coated plates as follows:
First step: 40 mm with CHCl3/MeOH/H2O (19:4:0.5 v/v/v).
Second step: 80 mm with hexane/diethyl ether/formic acid (14:6:0.2 v/v/v).
Plates were then dipped in an aqueous solution of copper sulfate, phosphoric acid 85%, ethanol, water (50:40:25:390 v/v/v/v) solution, then dried and heated for 8 min at 150 °C. The plates were scanned using CAMAG TLC scanner3 (Muttenz, Switzerland) at 500 nm.
Compounds were identified by comparing to authentic standards. Quantification was made using standards calibration curves. In these TLC migration conditions, SQDG, PC, and PG could not be analysed separately as they co-eluted.
2.2.7 Fatty acid composition
For this purpose, a methylation of the fatty acids of the Folch extracts used for the measurement of the fatty acid composition, (∼200 μL) previously evaporated under nitrogen was carried out according to the NF T30-233 standard with slight modifications. The oil extracts were added to 500 μL sodium methylate solution (0.5 M). Reaction medium was heated at 65 °C for 10 min. 500 μL hydrochloric acid in methanol (0.5 M) were added to phenolphthalein discoloration and the mixture was again heated at 65 °C for 10 min and then cooled to ambient temperature. Once the reaction mixture was cooled, 2 mL hexane and 2 mL water were added. After centrifugation 5 min at 1,500 rpm with a CR412 centrifuge (Jouan Thermo Electron, Waltham, USA), the organic phase was collected and analyzed by gas chromatography (GC). An 8860GC system Agilent (Agilent Technologies, Les Ullis, France) was used and equipped with a split injector (ratio of 1/20), a CP-Cil 88 Varian capillary column (50 m × 0.25 mm with 0.2 µm film thickness; Agilent Chrompack, Mid-delburg, Netherlands), and helium (flow rate: 1 mL min−1) as the carrier gas was used. Fatty acids methyl esters (FAME) were analyzed by flame ionization detector and Openlab software data system (version B.01.18, 2019, Agilent Technologies, Les Ullis, France). The column temperature started from 150 °C, then reached 150 to 225 °C with a rise of 5 °C min−1 and was kept at 225 °C for 10 min. The injector and detector temperatures were 250 and 270°C, respectively. FAME were identified using as external standards a mixture of methyl esters.
3 Results and discussion
3.1 Commercial biomasses
Due to the increasing interest of consumers in microalgae, a multitude of commercial biomasses is now available in the market. To our knowledge, the variability of commercial microalgae composition is barely described. In this study, six commercial biomasses were analyzed in terms of lipid content, lipid classes and fatty acid composition. Among them, four were identified as C. vulgaris, one as “C. pyrenoidosa” (not registered as a species anymore, (Champenois et al., 2015)) and one as C. sorokiniana. Their lipid content ranged from 5% to 15% of dry matter (DM), varying with the species, year of production and brand (Tab. 1). In most cases, the total lipid values obtained here (by weighing the lipid extract) were remotely superior to those labelled on the products, maybe because different quantification methods were used. Still, for the Cv_A-com biomass brand, the amount of lipids extracted in the 2022 batch was three times lower than in 2021 (5% vs 14%) while the description was unchanged (indicating 2.3% of lipids). Still, our results align with other studies, such as those of Bernaerts et al. (2018) who obtained 6.6% lipid content for Chlorella vulgaris produced in Portugal (Bernaerts et al., 2018), and of commercial biomasses examined by Canelli et al., (2020) that displayed 8 to 10% of fatty acids in their dry matter.
3.1.1 Lipid classes profiles
To examine lipid classes in microalgae, the analysis conducted here focused on galactolipids (MGDG, DGDG, SQDG), main phospholipids (PC, phosphatidylglycerol (PG) and PE), TAG and FFA, by comparing their relative abundance in total lipid extracts. For all biomasses, the results showed significant variations in the lipid composition (Fig. 1). Membrane lipids (MGDG, DGDG, SQDG, PC, PG and PE) were predominant in all the commercial products analysed (between 70% and 91% for Cv_A-com-21 and Cv_C-com-21, respectively) with MGDG and DGDG being the principal molecular forms (47% for both Cv_A-com-21 and Cs-com-21 and 68% for Cv_A-com-22). Notably, for the same biomass brand (Cv_A-com), the lipid composition also differed from one production to another: the MGDG content of Cv_A-com-22 was twice higher than in Cv_A-com-21 while TAG and FFA levels were higher in the latter. Storage lipid (TAG) content ranged from 1.6% in Cp-com-22 to 12.1% in Cv_B-com-21. The prevalence of membrane lipids on TAG in the commercial products suggested that no evident strategy targeting lipid storage were implemented for the production of these microalgae (Couto et al., 2021; White et al., 2019).
FFA content revealed additional disparities with content ranging between 1.4% for Cv_C-com-21 to 17.9% for Cv_A-com-21, indicating possible differences in the management of lipolysis in the products. For exemple, Balduyck et al. (2017, 2016) have shown that endogenous lipolysis could start just after the harvest of microalgae, at rates depending on the microalgae species, its cell wall potential damage, and on the storage temperature. While their study highlighted the key role of the integrity of the microalgal cell wall to limit endogenous hydrolysis activity in freshly harvested cells, our results allowed no correlation between FFA levels and cell wall breakage. Indeed, the commercial biomasses displaying the highest and lowest FFA levels both had broken cell walls. Thus, in the commercial products where FFA levels were substantial (>2%) lypolisis probably occurred before the drying and eventual breakage steps. If protective means can be are employed in laboratory experimentations to minimize lipolysis before extraction, such procedures are challenging to apply in large scale production. In the present study, the results releaved a significant variation in FFA content among commercial biomasses, with levels exceeding 10% in two out of the six biomasses tested. Unfortunately, FFA content in total lipid after harvesting is not often measured in the literature. Although cell toughness may vary upon the biotic and abiotic cultivation conditions, only very few studies focus on these questions, which are nonetheless essential when it comes to quality of the final product, especially regarding the released FFA content.
When considering microalgae biomass for human consumption, the presence of FFA must not be overlooked. The bioavailability and potential toxicity of FFA depend on factors such as the aliphatic chain’s length and the presence of unsaturation. For example, Michalski et al. (2020) demonstrated that only short chains of FFA could absorbed, while studies also indicated that ω3-FFA, like eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), exhibited higher bioavailability than ω3-TAG in humans, contrary to rats (Michalski et al., 2013; Punia et al., 2019). The molecular form of the FA is thus crutial for their assimilation, as well as the structure of the lipids. For instance, concerning ALA, Couëdelo et al. (2017) highlighted a better absorption of ALA when ALA-TAG were in emulsion and/or with phospholipids (Couëdelo et al., 2017). Otherwise, Robert et al. (2020) compared ALA vectorization under the form of glycerophospholipids or TAG and did not evidence, on the short term and at nutritional dose, impact of the molecular vector on postprandial lipemia or liver content in ALA, EPA or DHA. Today, while dietary lipids predominantly consist of TAG (∼80 g/d) (AFSSA, 2011)) and phospholipids (2–10 g/d) (Cohn et al., 2010), the consumption of galactolipids is increasing (∼0.2 g/d estimated by Sahaka et al. (2020)). With the growing emphasis on sustainable food sources, galactolipids from terrestrial (photosynthetic plant) or aquatic (microalgae) origin contribute to PUFA-ω3 intake, alongside TAG from seeds and nuts (Kergomard et al., 2021). The metabolic fate of such lipids is influenced by several structural factors at both molecular and supramolecular levels, such as fatty acyl moieties length, distribution on the TAG, PL or GL molecule, as well as the crystallinity and emulsified state (Michalski et al., 2013; Vors et al., 2020), all necessitating further exploration.
Altogether, our results illustrate the disparity in the quantity and molecular forms of lipids available in the commercial biomasses, with no consistency related to species or brand. While such differences are known to modulate the quality and biodiponibility of the lipids, the information available on the products is not explicit.
 |
Fig. 1 Lipid composition of Chlorella spp. commercial biomasses. |
3.1.2 FA profiles
The fatty acid composition of the six biomasses were analysed (Fig. 2). In all the commercial biomasses tested linoleic acid (LA, C18:2ω6) was the major fatty acid accounting for 30 to 46% of total FA (in Cp-com-22 and Cv_A-com-22, respectively), followed by palmitic acid (C16:0), and hexadecadienoic acid (C16:2 ω6) (HDA) acids. ALA content ranged between 5% and 9%, except in Cp-com-22 where it was notably high (21%). All the biomasses also contained C18:1, C16:1, C18:0, eventually C16:4, at level superior to 1%, and the major C16:1 isomer detected was C16:1 ω9 (cis-7-hexadecenoic acid), in line with the literature on Chlorella spp. cells where the major fatty acids are C18:2 and C16:0, and eventually C18:3, while C16:2, C16:3, C16:1, C18:1 C18:0 and C14:0 are also present in varying proportions (Otleş and Pire, 2001; Petkov and Garcia, 2007; Yun et al., 2020). The presence of fatty acids with odd numbers of carbon atoms, e.g. C15:0, C17:0 and C17:1, is now considered as coming from bacterial contamination and/or inaccurate identification of C16 PUFA (Petkov and Garcia, 2007), an assumption supported by the results obtained here. Interestingly, C16:4 also was identified in small amount (<1%) although rarely displayed in literature on Chlorella (Lin et al., 2022).
With these results, the ω6/ω3 ratio were calculated (corresponding to the [ω6 (LA + HDA)/ ω3 (ALA + RoA + C16:4ω3)] ratio in this study) as well as the LA/ALA ratio for comparison. In the commercial biomasses analysed here, the ω6/ω3 ratios ranged from 1.3 to 8.9 for Cp-com-22 and Cv_A-com-22, respectively and were lower than the LA/ALA ratios of which range was wider, from 1.4 to 10.4 for Cp-com-22 and Cv_C-com-21 respectively (Table 2). Depending on the FA used for calculation, the ratios were significantly different and did not result in the same ranking in between the samples. The lowest ω6/ω3 and LA/ALA ratios obtained (<1.5) were those of the Cp-com-22 for which the microalgae species label was unfortunately unaccurate (e.g. “C. pyrenoïdosa”). For the commercial brand A produced in 2021 and 2022 (Cv_A-com), the ω6/ω3 ratios were 5.2 and 8.9 respectively, showing significant variation depending on the production year. Considering only LA/ALA ratio, except for Cp-com-22, in average the ratios calculated here appeared higher in comparison with those obtained by Canelli et al. (2020) who showed LA/ALA ratios between 1.8 and 5. Surprisingly also, these authors did not identify any C16 PUFA (HDA and RoA) while they found significant amounts of C17:1 acid.
Again, if the FA profiles obtained here were in the ranges described in the litterature, they also illustrate the qualitative diversity that can be found in the commercial products. Regarding specifically the ω6/ω3 ratio, the fact that it can be superior or inferior to the 4/1 target (AFSSA, 2011; Legrand, Philippe, 2013) shall be considered for product labelling. So far, the information related to lipids available on the product gives at best the proportions of SFA, PUFA and eventually MUFA (Tab. 1). As stated before, although it is known that FA profile depend on the biomass production methods (T. Li et al., 2014; Wan et al., 2012; Yun et al., 2021), no indication related to the production process is given on the packaging.
 |
Fig. 2 Fatty acid composition (%) of Chlorella spp. commercial biomasses. |
Ratios of ω6 (LA + HDA)/ω3 (ALA + RoA + C16:4 ω3) and LA/ALA calculated from fatty acyl profiles of Chlorella commercial biomasses.
3.2 In-lab biomasses
Lipid classes profiles and fatty acid composition of microalgae biomass are influenced by the abiotic conditions of culture and storage, information often undisclosed on product labels. To elucidate specifically the possible correlation with the tropic mode, the commercial products were compared with controlled in lab-production of C. sorokiniana grown in photo-autotrophy and mixotrophy with glucose. Since no significant TAG accumulation was observed in the commercial biomasses, heterotrophy was not tested (Couto et al., 2021; Yun et al., 2021). The growth was stopped at the beginning of stationary phase, when optical densities became stable (OD of 5 for photo-autotrophic cultures reached in 7 days, and between 6 and 6.5 for mixotrophic cultures reached in 3–4 days). The addition of glucose allowed a significant increase of the biomass production, especially from 0 to 5 g/L with 0.8 g dry matter (DM)/L in photo-autotrophy and 2.4 g DM/L in mixotrophy with 5 g/L of glucose, respectively (Tab. 3).
C. sorokiniana grown in photo-autotrophy exhibited the highest total lipid content in dry matter (22%). In mixotrophy with glucose, in lab-produced cells with 2 g/L glucose had the higher total lipid content with 20%, whereas the lowest level (13%) was obtained in the biomass produced with a glucose concentration of 5 and 8 g/L, showing that the relative lipid content decreased when the biomass production increased. Here, the global effect of the trophic mode on dry matter and lipid yield obtained were in accordance with results obtained by other researchers, although the absolute values were not always fully consistent. For instance, Juntila et al. (2015) demonstrated the beneficial effect of adding glucose on the growth of C. sorokiniana in mixotrophic conditions on BBM basal medium, but the dry matter (DM) of biomass they obtained were always inferior to 0.7 g/L. The authors obtained lipid content ranging from 28% to 20% DM with glucose at 0.5 to 2 g/L and high lipid content was also obtained in photo-autotrophic condition (28%). T. Li et al. (2014) studied mixotrophic cultivation of C. sorokiniana (UTEX 1602) in Kuhl basal medium, obtaining comparable trends but up to 5 g DM/L with 8 and 10 g/L of glucose. Their biomass exhibited higher lipid content with glucose concentration between 4 and 8 g/L (e.g. 31.5% of DM in mixotrophy with glucose at 6 g/L) while in photoautrophic cultivation the lipid content was lower with 6.65% in a biomass reaching 0.47 g/L. Thus, besides the significant impact of the trophic mode on the DM and lipid content, additional abiotic parameters such as basal medium composition and physical parameters (illumination, temperature) also influence the lipid quality of the product. For example, Wan et al. (2012) cultivated a different strain of C. sorokiniana (CCTCC M209220) in photo-autotrophy and heterotrophy on a GB11 basal medium and observed that biomass and lipid yields were strongly dependent on temperature and nitrogen source. The authors achieved an average lipid content of 19% in the photo-autotrophic condition (sodium nitrate as nitrogen source, 14:10 light/dark photoperiod), close to ours. Their strain displayed high ability to consume glucose in heterotrophy, reaching a maximum lipid content of over 50% with glucose concentrations ranging from 10 to 60 g/L. Chai et al. (2018) also cultivated C. sorokiniana UTEX 1230 in autotrophy, mixotrophy (14:10h light/dark photoperiod) and heterotrophy (dark) on a BBM basal medium with different organic carbon sources. In mixotrophy and heterotrophy, they showed that both biomass and lipid concentrations increased with that of glucose, with residual glucose remaining after 7 days when initial concentration was above 4 g/L. From their data, the mass percent of lipid was estimated in the dry biomass between 8 to 18% for an initial glucose concentration at 0 to 16 g/L. Therefore, and in opposition with the results of this study, in their conditions (another strain and photoperiods) the lipid content in the biomass (%) increased with increasing glucose concentration in mixotrophy (and heterotrophy). In the literature, the usage of various biotic and abiotic conditions explains the discrepancies that can be found in the results, but also highlights the difficulty to extrapolate these results to industrial process productions.
Summary of in-lab cultivations of C. sorokiniana: trophic mode, final biomass dry weight obtained at the beginning of the stationary phase, final lipid yield and Ratios of ω6 (LA + HDA)/ω3 (ALA + RoA + C16:4 ω3) and LA/ALA calculated from fatty acyl profiles.
3.2.1 Lipid classes profiles
The same classes of lipids were identified by TLC in the biomass of C. sorokiniana produced in lab as in the commercial biomasses: phospholipids (PC, PE and PG), galactolipids (MGDG, DGDG, SDQG) and non-polar lipids (FFA and TAG) (Fig. 3). Notably, diacylglyceryl-trimethylhomoserine (DGTS) was not detected in the conditions used, although some studies reported its presence in Chlorella sp. (Couto et al., 2023; S. Li et al., 2014; White et al., 2019). The results from in-lab cultivations confirmed that different growth conditions significantly affected the relative composition of the lipid species, mainly regarding the galactolipids vs non-polar lipids ratio. The autotrophy condition promoted MGDG (61%) whereas the mixotrophy condition boosted the TAG: 0 for autotrophy vs 30–42% for mixotrophy conditions. In the mixotrophy conditions, increasing glucose content from 2 to 8 g/L decreased the proportion of TAG and whiled increased proportion of FFA were observed. For the growth condition at 10 g/L glucose, the amount of MGDG was the higher of all the mixotrophy conditions. Hence, in photo-autotrophy, the total lipid content of the biomass was maximized and mostly composed of galactolipids (84%) which is consistent with the literature since galactolipids are the major component of thylakoid membranes. Within the conditions tested, mixotrophy shall be preferred to favor TAG accumulation, with 2 g/L of glucose appearing optimal to also maximize total lipid content in the biomass (Yeh and Chang, 2012). In comparison with the results obtained on the commercial biomasses, the closest lipid profile obtained in lab was observed in photo-autotrophy cultures.
Since strains and cultivation conditions are never exactly identical in the literature, it is not easy to accurately compare the results obtained by different authors or to extrapolate those obtained in lab to commercial productions. Still, a general trend, confirmed by our results in laboratory, is that photo-autotrophy favors the accumulation of galactoplids, themselves enriched in PUFA, while biomass produced in mixotrophy in the presence of an organic carbon source increases the proportion of phospholipids and non-polar lipids (Couto et al., 2023; Wan et al., 2012). White et al. (2019) also demonstrated that the ratio between the different lipid classes was shown to depend strongly on the growth stage of the microalgae. This illustrates that the lipid composition of the biomass is influenced not only by the strain, the trophic mode, the medium composition, the illumination conditions, but also by the timing of harvesting during growth. Thus, depending on the specific molecular class of lipid targeted, studies need to be conducted under production conditions in order to tailor optimal lipid content and composition.
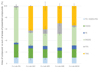 |
Fig. 3 Lipid composition of C. sorokiniana in lab cultivated in different trophic mode: Photo-Autotrophy (Cs-Lab-PA), Mixotrophy with glucose at 2 g/L (Cs-Lab-M2), 5 g/L (Cs-Lab-M5), 8 g/L (Cs-Lab-M8), 10 g/L (Cs-Lab-M10). |
3.2.2 FA profiles
The impact of both autotrophy and mixotrophy conditions on the fatty acid composition were also analyzed (Fig. 4). Although the major acyl chains remained the same, significant qualitative differences were observed. Regardless of the growth condition, LA was the most abundant (23–34%) followed by palmitic acid (C16:0) (20-25%). ALA contents ranged from 7 to 15% for Cs-Lab-M5/Cs-Lab-M8 and Cs-Lab-PA conditions, respectively. Comparing photo-autotrophy and mixotrophy modes, the highest differences in fatty acids percentages were observed for the HDA (C16:2ω6) and ALA. In photo-autotrophy and mixotrophy, the contents of HDA and ALA were 14 vs 8 % and 15 vs 8%, respectively. Increasing the glucose content in mixotrophy did not seem to affect significantly the HDA and ALA content. Therefore, the ω6/ω3 ratios varied between 2.4 and 4.4 (Tab. 3), with the minimum obtained in photo-autotrophy condition while the highest was reached in mixotrophy in the presence of 2 g/L of glucose. Here the LA/ALA ratio was lower than the ω6/ω3 ratio in photo-autotrophy and mixotrophy with 2 g/L and 10 g/L of glucose, and higher in the two other mixotrophic conditions. The results thus demonstrated again a difference of ranking between the samples depending on the FA considered for calculation, although the best condition to minimize the ratios was still photo-autotrophy. Globally, the results were in accordance with the literature, confirming the importance of the trophic mode for FA relative content, especially for the ω6/ω3 ratio, with photo-autotrophy being the most appropriate cultivation condition to lower the ω6/ω3 ratio.
Together with the fact that, in mixotrophy, glucose addition increased the proportion of TAG vs membrane lipids (Fig. 3), our results also confirmed that the usual strategies used to increase lipid content under the form of storage lipids, e.g. TAG, are not ideal to increase PUFA proportions. Reflecting this, the study of Yeh and Chang (2012) revealed a distinct pattern wherein microalgal cells (C. vulgaris ESP-31) displaying lipid content exceeding 40% exhibited higher proportions of C16:0 and C18:1 in their lipids. Conversely, microalgal lipids with content below 30% tended to feature more C16:0 and C18:2 fatty acids.
In addition to the consideration of the fatty acids molecular classes in the biomass and their relationships to bioaccessibility, the fatty acid profile is also essential for the nutritional benefits. To evaluate the lipid nutritional quality of the product, the ratios between saturated, unsaturated and polyunsaturated fatty acids, and between PUFA ω6 and ω3 are required. In this study, the ω6/ω3 ratio varied between 1.3 and 8.9 in commercial biomasses of different strains, and between 2.4 and 4.4 in the same strain of C. sorokiniana cultivated in photo-autotrophy and mixotrophy, respectively. In the literature, this ratio is also significantly variable and depends not only on the experimental procedures but also, on the fatty acyl chain identified and considered for the calculation. As mentioned earlier, only LA for ω6 and ALA for ω3 are often used for the determination of the ω6/ω3 ratio (Canelli et al., 2020), while C16:2 to LA for ω6, and RoA (C16:3ω3) and C16:4ω3 to ALA for ω3 were added here. For a more accurate comparison with the results of this work, the [ω6 (LA + C16:2)/ω3 (ALA + RoA + C16:4ω3)] ratio obtained from literature data were computed. Globally, Chlorella spp. exhibited low ω6/ω3 ratios, especially for cultivation in photo-autotrophy. For example, in strains of C. vulgaris and/or C. sorokiniana, ratios of 0.6-0.9 were calculated for cultivation in autotrophy, of 1.3-1.6 in mixotrophy (Couto et al., 2021; Yun et al., 2021, Yun et al., 2020) and up to 3.2 in heterotrophy (Couto et al., 2021). Abiotic factors such as temperature were shown to increase the ω6/ω3 ratio (Yun et al., 2020). Taking into account all these parameters is quite challenging but nevertheless needed for clearer evaluation of microalgae consumption benefits.
Ultimately, not only the fatty acid composition and the lipid classes are impacted by abiotic parameters but also compounds of interest like vitamins (for instance carotenoids, tocopherols) or phenolic contents. Canelli et al. (2022) highlighted that the combination of mixo- and heterotrophic cultivation raised carotenoid contents, while fatty acids and tocopherols contents increased in cultures depleted in nitrogen. The authors showed also that the bioaccessibility of these molecules of interest was enhanced by 12 (up to 76%) with either a pulsed electric field or high-pressure homogenization step.
 |
Fig. 4 Fatty acid profile of C. sorokiniana in lab cultivated in different trophic mode: Photo-Autotrophy (Cs-Lab-PA), Mixotrophy with glucose at 2 g/L (Cs-Lab-M2), 5 g/L (Cs-Lab-M5), 8 g/L (Cs-Lab-M8), 10 g/L (Cs-Lab-M10). |
4 Conclusion and perspectives
In this study, the cultivation of the C. sorokiniana strain on a BBM basal medium under photo-autotrophic conditions (at 25 °C, pH 7, under constant illumination) has been identified as an effective trophic mode for targeting glycolipids (MGDG and DGDG) and the accumulation of polyunsaturated fatty acids (PUFA). Conversely, when the focus is on non-polar lipid (TAG) accumulation, mixotrophy with a low glucose concentration (2 g/L) has been proven optimal for maximizing both biomass and lipid yields. Our findings confirm previously published data, emphasizing the dependency of absolute and relative PUFA abundance, particularly the ω6/ω3 ratio, on biotic and abiotic conditions but adds on knowledge considering the impact of trophic mode on the non polar/polar lipid ratio.
Furthermore, our study underscores the significant variations in lipid profiles among commercial biomasses of Chlorella spp., even within the same species and the same commercial brand. This variability highlights the importance of implementing better controls over lipid content and quality in commercial Chlorella products to ensure consistent quality, both in terms of available molecular classes and ω6 to ω3 content. Microalgae producers should prioritize a deeper understanding of these factors to guarantee the quality and reproducibility of lipid profiles in their products. Achieving an optimal lipid profile requires meticulous control of cultivation conditions, with downstream processing also demanding careful monitoring to preserve lipid quality in relation to cell integrity and potential lipolysis. Finally, the nutritional quality of microalgae lipids could be managed through cultivation and processing and this point should be addressed in future studies. Indeed, for a given microalgae species, depending on the expected effect, i.e. fatty acid composition, ω6/ω3 ratio, polar lipid content, a lot of strategies can be set up to achieve the best compromise in terms of nutritional intake and bioaccessibility of the bioactive molecules (Legrand, 2013; Vors et al., 2020). Last but not the least, the presence and efficiency of antioxidants like tocopherols or carotenoids to protect against oxidation should not be overlooked as they are considered as key bioactive molecules in microalgae as it has been highlighted in recent preclinical or clinical studies (Neumann et al., 2019, 2018; Stiefvatter et al., 2021).
Abbreviation
Cv_A-com-21: C. vulgaris brand A-commercial 2021. Broken cell wall
Cv_A-com-22: C. vulgaris brand A-commercial - 2022. Broken cell wall
Cv_B-com-21: C. vulgaris brand B-commercial- 2021. Whole cells
Cv_C-com-22: C. vulgaris c brand C-commercial- 2021. Whole cells
Cp-com-22: C. pyrenoidosa commercial-2022. Broken cell wall
Cs-com-21: C. sorokiniana commercial-2021. Broken cell wall “fermented”
Cs-Lab-PA: in-lab produced C. sorokiniana grown in photo-autotrophy
Cs-Lab-M2: in-lab produced C. sorokiniana grown in mixotrophy with 2 g/L glucose)
Cs-Lab-M5: in-lab produced C. sorokiniana grown in mixotrophy with 5 g/L glucose)
Cs-Lab-M8: in-lab produced C. sorokiniana grown in mixotrophy with 8 g/L glucose)
Cs-Lab-M10: in-lab produced C. sorokiniana grown in mixotrophy with 10 g/L glucose)
DGDG:: digalactosyldiacylglycerol
DGTS: diacylglyceryl-trimethylhomoserine
FFA: free fatty acid (also named NEFA for non esterified fatty acid)
MGDG: monogalactosyldiacylglycerol
RoA: hexadecatrienoic acid (roughanic acid)
SQDG: sulfoquinovosyldiacylglycerol
TLC: thin layer chromatography
Conflicts of Interest
The authors declare that they have no conflict of interest in relation to this article.
Author contribution statement
Nathalie Barouh: methodology, investigation, writing- original draft.
Juliette Wind: investigation, Writing −original draft.
Victoria Chuat: Writing − review & editing.
Valérie Gagnaire: Writing − review & editing.
Florence Valence: Writing − review & editing.
Claire Bourlieu-Lacanal: conceptualization, methodology, supervision, writing-review & editing.
Maeva Subileau: conceptualization, methodology, supervision, writing- original draft, writing-review & editing.
References
- AFSSA. 2011. Avis de l’Agence française de sécurité sanitaire des aliments relatif à l’actualisation des apports nutritionnels conseillés (ANC) pour les acides gras. AFSSA − Saisine n° 2006–SA-0359 − Avis 1er mars et Rapport ANSES mai 2011. [Google Scholar]
- Araujo R, Peteiro C. 2021. Algae as food and food supplements in Europe. Publications Office of the European Union, Luxembourg. [Google Scholar]
- Araújo R, Vázquez Calderón F, Sánchez López J, Azevedo IC, Bruhn A, Fluch S, Garcia Tasende M, Ghaderiardakani F, Ilmjärv T, Laurans M, Mac Monagail M, Mangini S, Peteiro C, Rebours C, Stefansson T, Ullmann J. 2021. Current status of the algae production industry in europe: an emerging sector of the blue bioeconomy. Front Mar Sci. 7. https://doi.org/10.3389/fmars.2020.626389 [Google Scholar]
- Balduyck L, Bijttebier S, Bruneel C, Jacobs G, Voorspoels S, Van Durme J, Muylaert K, Foubert I. 2016. Lipolysis in T-Isochrysis lutea during wet storage at different temperatures. Algal Res 18: 281–287. https://doi.org/10.1016/j.algal.2016.07.003 [CrossRef] [Google Scholar]
- Balduyck L, Stock T, Bijttebier S, Bruneel C, Jacobs G, Voorspoels S, Muylaert K, Foubert I. 2017. Integrity of the microalgal cell plays a major role in the lipolytic stability during wet storage. Algal Res 25: 516–524. https://doi.org/10.1016/j.algal.2017.06.013 [CrossRef] [Google Scholar]
- Bernaerts TMM, Gheysen L, Kyomugasho C, Kermani ZJ, Vandionant S, Foubert I, Hendrickx ME, Loey AMV. 2018. Comparison of microalgal biomasses as functional food ingredients: focus on the composition of cell wall related polysaccharides. Algal Res 32: 150–161. https://doi.org/10.1016/j.algal.2018.03.017 [CrossRef] [Google Scholar]
- Bischoff HW, Bold HC. 1963. IV. Some soil algae from Enchanted Rock and related algal species, in: Phycological Studies, University of Texas Publication. [Google Scholar]
- Bito T, Okumura E, Fujishima M, Watanabe F. 2020. Potential of Chlorella as a dietary supplement to promote human health. Nutrients 12: 2524. https://doi.org/10.3390/nu12092524 [CrossRef] [PubMed] [Google Scholar]
- Bold HC. 1949. The morphology of Chlamydomonas chlamydogama, Sp. Nov. Bull Torrey Bot Club 76: 101. https://doi.org/10.2307/2482218 [CrossRef] [Google Scholar]
- Canelli G, Tarnutzer C, Carpine R, Neutsch L, Bolten CJ, Dionisi F, Mathys A. 2020. Biochemical and nutritional evaluation of chlorella and auxenochlorella biomasses relevant for food application. Front Nutr 7. https://doi.org/10.3389/fnut.2020.565996 [CrossRef] [PubMed] [Google Scholar]
- Canelli G, Tevere S, Jaquenod L, Dionisi F, Rohfritsch Z, Bolten CJ, Neutsch L, Mathys A. 2022. A novel strategy to simultaneously enhance bioaccessible lipids and antioxidants in hetero/mixotrophic Chlorella vulgaris as functional ingredient. Bioresour Technol 347: 126744. https://doi.org/10.1016/j.biortech.2022.126744 [CrossRef] [PubMed] [Google Scholar]
- Chai S, Shi J, Huang T, Guo Y, Wei J, Guo M, Li L, Dou S, Liu L, Liu G. 2018. Characterization of Chlorella sorokiniana growth properties in monosaccharide-supplemented batch culture. PLOS ONE 13: e0199873. https://doi.org/10.1371/journal.pone.0199873 [CrossRef] [PubMed] [Google Scholar]
- Champenois J, Marfaing H, Pierre R. 2015. Review of the taxonomic revision of Chlorella and consequences for its food uses in Europe. J Appl Phycol 27: 1845–1851. https://doi.org/10.1007/s10811-014- 0431-2 [CrossRef] [Google Scholar]
- Cohn JS, Kamili A, Wat E, Chung RW, Tandy S. 2010. Dietary phospholipids and intestinal cholesterol absorption. Nutrients 2: 116–127. https://doi.org/10.3390/nu2020116 [CrossRef] [PubMed] [Google Scholar]
- Couëdelo L, Termon A, Vaysse C. 2017. Matrice lipidique et biodisponibilité de l’acide alpha-linolénique. OCL 24. https://doi.org/10.1051/ocl/2017005 [Google Scholar]
- Couto D, Conde TA, Melo T, Neves B, Costa M, Silva J, Domingues R, Domingues P. 2023. The chemodiversity of polar lipidomes of microalgae from different taxa. Algal Res 70: 103006. https://doi.org/10.1016/j.algal.2023.103006 [CrossRef] [Google Scholar]
- Couto D, Melo T, Conde TA, Costa M, Silva J, Domingues MRM, Domingues P. 2021. Chemoplasticity of the polar lipid profile of the microalgae Chlorella vulgaris grown under heterotrophic and autotrophic conditions. Algal Res 53: 102128. https://doi.org/10.1016/j.algal.2020.102128 [CrossRef] [Google Scholar]
- Couto D, Melo T, Conde TA, Moreira, A.S.P., Ferreira P, Costa M, Silva J, Domingues R, Domingues P. 2022. Food grade extraction of Chlorella vulgaris polar lipids: a comparative lipidomic study. Food Chem 375: 131685. https://doi.org/10.1016/j.foodchem.2021.131685 [CrossRef] [PubMed] [Google Scholar]
- Cuellar‐Bermudez SP, Aguilar‐Hernandez I,Cardenas‐Chavez DL, Ornelas‐Soto N, Romero‐Ogawa MA, Parra‐Saldivar R. 2015. Extraction and purification of high‐value metabolites from microalgae: essential lipids, astaxanthin and phycobiliproteins. Microb Biotechnol 8: 190–209. https://doi.org/10.1111/ 1751-7915. 12167 [CrossRef] [PubMed] [Google Scholar]
- Directive 2002 /46/CE du Parlement européen et du Conseil du 10 juin 2002 relative au rapprochement des législations des États membres concernant les compléments alimentaires [Google Scholar]
- Fernández FGA, Reis A, Wijffels RH, Barbosa M, Verdelho V, Llamas B. 2021. The role of microalgae in the bioeconomy. New Biotechnol 61: 99–107. https://doi.org/10.1016/j.nbt.2020.11.011 [CrossRef] [Google Scholar]
- Ferreira de Oliveira AP, Bragotto APA. 2022. Microalgae-based products: food and public health. Future Foods 6: 100157. https://doi.org/10.1016/j.fufo.2022.100157 [CrossRef] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497–509. [CrossRef] [PubMed] [Google Scholar]
- Johnson K, Ellis G, Toothill C. 1977. The sulfophosphovanillin reaction for serum lipids: a reappraisal.Clin Chem 23: 1669–1678. [CrossRef] [PubMed] [Google Scholar]
- Jones J, Manning S, Montoya M, Keller K, Poenie M. 2012. Extraction of algal lipids and their analysis by HPLC and mass spectrometry. J Am Oil Chem Soc https://doi.org/10.1007/s11746-012- 2044-8 [Google Scholar]
- Juntila DJ, Bautista MA, Monotilla W. 2015. Biomass and lipid production of a local isolate Chlorella sorokiniana under mixotrophic growth conditions. Bioresour Technol 191: 395–398. https://doi.org/10.1016/j.biortech.2015.03.098 [CrossRef] [PubMed] [Google Scholar]
- Katiyar R, Arora A. 2020. Health promoting functional lipids from microalgae pool: a review. Algal Res 46: 101800. https://doi.org/10.1016/j.algal.2020.101800 [CrossRef] [Google Scholar]
- Kergomard J, Carrière F, Barouh N, Villeneuve P, Vié V, Bourlieu C. 2021. Digestibility and oxidative stability of plant lipid assemblies: an underexplored source of potentially bioactive surfactants? Crit Rev Food Sci Nutr 1–20. https://doi.org/10.1080/10408398.2021.2005532 [Google Scholar]
- Kiran BR, Venkata Mohan S. 2021. Microalgal cell biofactory—therapeutic, nutraceutical and functional food applications. Plants 10: 836. https://doi.org/10.3390/plants10050836 [CrossRef] [PubMed] [Google Scholar]
- Kumar R, Hegde AS, Sharma K, Parmar P, Srivatsan V. 2022. Microalgae as a sustainable source of edible proteins and bioactive peptides − current trends and future prospects. Food Res Int 157: 111338. https://doi.org/10.1016/j.foodres.2022.111338 [CrossRef] [PubMed] [Google Scholar]
- Legrand P. 2013. Nouvelle approche pour les recommandations nutritionnelles en lipides. OCL 20: 75–78. https://doi.org/10.1051/ocl.2013.0502 [CrossRef] [EDP Sciences] [Google Scholar]
- Li S, Xu J, Chen J, Chen J, Zhou C, Yan X. 2014. The major lipid changes of some important diet microalgae during the entire growth phase. Aquaculture 428-429: 104–110. https://doi.org/10.1016/j.aquaculture.2014.02.032 [CrossRef] [Google Scholar]
- Li T, Zheng Y, Yu L, Chen S. 2014. Mixotrophic cultivation of a Chlorella sorokiniana strain for enhanced biomass and lipid production. Biomass Bioenergy 66: 204–213. https://doi.org/10.1016/j.biombioe.2014.04.010 [CrossRef] [Google Scholar]
- Lin Y, Dai Y, Xu W, Wu X, Li Y, Zhu H, Zhou H. 2022. The growth, lipid accumulation and fatty acid profile analysis by abscisic acid and indol-3-acetic acid induced in Chlorella sp. FACHB-8. Int J Mol Sci 23: 4064. https://doi.org/10.3390/ijms23074064 [CrossRef] [Google Scholar]
- Michalski M-C., Couëdelo L, Penhoat A, Vaysse C, Vors C. 2020. Bioavailability and metabolism of dietary lipids, in: Lipids and Edible Oils. Elsevier, pp. 45–92. https://doi.org/10.1016/B978-0-12 - 817105–9. 00002–1 [Google Scholar]
- Michalski M-C., Genot C, Gayet C, Lopez C, Fine F, Joffre F, Vendeuvre J-L., Bouvier J, Chardigny J-M., Raynal-Ljutovac K. 2013. Multiscale structures of lipids in foods as parameters affecting fatty acid bioavailability and lipid metabolism. Prog Lipid Res 52: 354–373. https://doi.org/10.1016/j.plipres.2013.04.004 [CrossRef] [PubMed] [Google Scholar]
- Mishra SK, Suh WI, Farooq W, Moon M, Shrivastav A, Park MS, Yang J-W. 2014. Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour Technol 155: 330–333. https://doi.org/10.1016/j.biortech.2013.12.077 [CrossRef] [PubMed] [Google Scholar]
- Mobin S, Alam F. 2017. Some promising microalgal species for commercial applications: A review. Energy Proc 110: 510–517. https://doi.org/10.1016/j.egypro.2017.03.177 [CrossRef] [Google Scholar]
- Neumann U, Derwenskus F, Flaiz Flister V, Schmid-Staiger U, Hirth T, Bischoff SC. 2019. Fucoxanthin, a carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities in vitro. Antioxidants 8: 183. https://doi.org/10.3390/antiox8060183 [CrossRef] [PubMed] [Google Scholar]
- Neumann U, Derwenskus F, Gille A, Louis S, Schmid-Staiger U, Briviba K, Bischoff SC. 2018. Bioavailability and safety of nutrients from the microalgae Chlorella vulgaris, Nannochloropsis oceanica and Phaeodactylum tricornutum in C57BL/6 mice. Nutrients 10: 965. https://doi.org/10.3390/nu10080965 [CrossRef] [PubMed] [Google Scholar]
- Otleş S, Pire R. 2001. Fatty acid composition of Chlorella and Spirulina microalgae species. J AOAC Int 84: 1708–1714. https://doi.org/10.1093/jaoac/84.6.1708 [CrossRef] [PubMed] [Google Scholar]
- Petkov G, Garcia G. 2007. Which are fatty acids of the green alga Chlorella? Biochem Syst Ecol 35: 281–285. https://doi.org/10.1016/j.bse.2006.10.017 [CrossRef] [Google Scholar]
- Petroutsos D, Amiar S, Abida H, Dolch L-J., Bastien O, Rébeillé F, Jouhet J, Falconet D, Block MA, McFadden GI, Bowler C, Botté C, Maréchal E. 2014. Evolution of galactoglycerolipid biosynthetic pathways-from cyanobacteria to primary plastids and from primary to secondary plastids. Prog Lipid Res 54: 68–85. https://doi.org/10.1016/j.plipres.2014.02.001 [CrossRef] [PubMed] [Google Scholar]
- Punia S, Sandhu KS, Siroha AK, Dhull SB. 2019. Omega 3-metabolism, absorption, bioavailability and health benefits − A review. Pharma Nutr 10: 100162. https://doi.org/10.1016/j.phanu.2019.100162 [Google Scholar]
- Robert C, Couëdelo L, Knibbe C, Fonseca L, Buisson C, Errazuriz-Cerda E, Meugnier E, Loizon E, Vaysse C, Michalski M-C. 2020. Rapeseed lecithin increases lymphatic lipid output and α-linolenic acid bioavailability in rats. J Nutr 150: 2900–2911. https://doi.org/10.1093/jn/nxaa244 [CrossRef] [PubMed] [Google Scholar]
- Sahaka M, Amara S, Wattanakul J, Gedi MA, Aldai N, Parsiegla G, Lecomte J, Christeller JT, Gray D, Gontero B, Villeneuve P, Carrière F. 2020. The digestion of galactolipids and its ubiquitous function in Nature for the uptake of the essential α-linolenic acid. Food Funct 11: 6710–6744. https://doi.org/10.1039/D0FO01040E [CrossRef] [PubMed] [Google Scholar]
- Saini RK, Keum Y-S. 2018. Omega-3 and omega-6 polyunsaturated fatty acids: dietary sources, metabolism, and significance − a review. Life Sci 203: 255–267. https://doi.org/10.1016/j.lfs.2018.04.049 [CrossRef] [PubMed] [Google Scholar]
- Sherafati N, Bideshki MV, Behzadi M, Mobarak S, Asadi M, Sadeghi O. 2022. Effect of supplementation with Chlorella vulgaris on lipid profile in adults: a systematic review and dose-response meta-analysis of randomized controlled trials. Complement Ther Med 66: 102822. https://doi.org/10.1016/j.ctim.2022.102822 [CrossRef] [PubMed] [Google Scholar]
- Simopoulos AP. 2016. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 8: 128. https://doi.org/10.3390/nu8030128 [CrossRef] [PubMed] [Google Scholar]
- Stiefvatter L, Lehnert K, Frick K, Montoya-Arroyo A, Frank J, Vetter W, Schmid-Staiger U, Bischoff SC. 2021. Oral bioavailability of omega-3 fatty acids and carotenoids from the microalgae Phaeodactylum tricornutum in healthy young adults. Mar Drugs 19. https://doi.org/10.3390/md19120700 [CrossRef] [PubMed] [Google Scholar]
- Vors C, Le Barz M, Bourlieu C, Michalski M-C. 2020. Dietary lipids and cardiometabolic health: a new vision of structure-activity relationship. Curr Opin Clin Nutr Metab Care 23: 451–459. https://doi.org/10.1097/MCO 0000000000000693 [CrossRef] [PubMed] [Google Scholar]
- Wan M-X., Wang R-M., Xia J-L., Rosenberg JN, Nie Z-Y., Kobayashi N, Oyler GA, Betenbaugh MJ. 2012. Physiological evaluation of a new Chlorella sorokiniana isolate for its biomass production and lipid accumulation in photoautotrophic and heterotrophic cultures. Biotechnol Bioeng 109: 1958–1964. https://doi.org/10.1002/bit.24477 [CrossRef] [PubMed] [Google Scholar]
- White DA, Rooks PA, Kimmance S, Tait K, Jones M, Tarran GA, Cook C, Llewellyn CA. 2019. Modulation of polar lipid profiles in Chlorella sp. in response to nutrient limitation. Metabolites 9. https://doi.org/10.3390/metabo9030039 [CrossRef] [PubMed] [Google Scholar]
- Wong JF, Hong HJ, Foo SC, Yap, MKK, Tan JW. 2022. A review on current and future advancements for commercialized microalgae species. Food Sci Hum Wellness 11: 1156–1170. https://doi.org/10.1016/j.fshw.2022.04.007 [CrossRef] [Google Scholar]
- Yeh K-L., Chang J-S.2012. Effects of cultivation conditions and media composition on cell growth and lipid productivity of indigenous microalga Chlorella vulgaris ESP-31. Bioresour Technol 105: 120–127. https://doi.org/10.1016/j.biortech.2011.11.103 [CrossRef] [PubMed] [Google Scholar]
- Yun H-S., Kim Y-S., Yoon H-S. 2021. Effect of different cultivation modes (photoautotrophic, mixotrophic, and heterotrophic) on the growth of Chlorella sp. and Biocompositions.Front Bioeng Biotechnol 9: 774143. https://doi.org/10.3389/fbioe.2021.774143 [CrossRef] [PubMed] [Google Scholar]
- Yun H-S., Kim Y-S., Yoon H-S. 2020. Characterization of Chlorella sorokiniana and Chlorella vulgaris fatty acid components under a wide range of light intensity and growth temperature for their use as biological resources. Heliyon 6: e04447. https://doi.org/10.1016/j.heliyon.2020.e04447 [CrossRef] [PubMed] [Google Scholar]
Cite this article as: Barouh N, Wind J, Chuat V, Gagnaire V, Valence F, Bourlieu-Lacanal C, Subileau M. 2024. Variations in Chlorella lipid content in commercial and in-lab produced biomass. OCL 31: 9.
All Tables
Ratios of ω6 (LA + HDA)/ω3 (ALA + RoA + C16:4 ω3) and LA/ALA calculated from fatty acyl profiles of Chlorella commercial biomasses.
Summary of in-lab cultivations of C. sorokiniana: trophic mode, final biomass dry weight obtained at the beginning of the stationary phase, final lipid yield and Ratios of ω6 (LA + HDA)/ω3 (ALA + RoA + C16:4 ω3) and LA/ALA calculated from fatty acyl profiles.
All Figures
 |
Fig. 1 Lipid composition of Chlorella spp. commercial biomasses. |
| In the text | |
 |
Fig. 2 Fatty acid composition (%) of Chlorella spp. commercial biomasses. |
| In the text | |
 |
Fig. 3 Lipid composition of C. sorokiniana in lab cultivated in different trophic mode: Photo-Autotrophy (Cs-Lab-PA), Mixotrophy with glucose at 2 g/L (Cs-Lab-M2), 5 g/L (Cs-Lab-M5), 8 g/L (Cs-Lab-M8), 10 g/L (Cs-Lab-M10). |
| In the text | |
 |
Fig. 4 Fatty acid profile of C. sorokiniana in lab cultivated in different trophic mode: Photo-Autotrophy (Cs-Lab-PA), Mixotrophy with glucose at 2 g/L (Cs-Lab-M2), 5 g/L (Cs-Lab-M5), 8 g/L (Cs-Lab-M8), 10 g/L (Cs-Lab-M10). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.




