| Issue |
OCL
Volume 20, Number 1, January-February 2013
|
|
|---|---|---|
| Page(s) | 16 - 22 | |
| Section | Dossier : Chimie du végétal et lipochimie | |
| DOI | https://doi.org/10.1051/ocl.2012.0489 | |
| Published online | 15 January 2013 | |
Synthesis of bio-based building blocks from vegetable oils: a platform chemicals approach
Institut Charles Gerhardt UMR CNRS 5253 Equipe Ingénierie et Architecture Macromoléculaire, Ecole Nationale Supérieure de Chimie de Montpellier, 8 rue de l’Ecole Normale, 34296 Montpellier Cedex 05, France
Received:
26
October
2012
Accepted:
6
November
2012
This review reports the synthesis of various building blocks from vegetable oils in one or two-steps syntheses. Thiol-ene coupling allows to synthesize new biobased reactants with various function and functionality with reaction conditions in agreement with green chemistry principles: it does not use neither solvent nor initiator or need simple purification step, feasible at industrial scale. Esterification and amidification were also used to insert ester or amide groups in fatty chains in order to modifiy properties of thereof synthesized polymers. Building blocks synthesized have various functions and functionality: polyols, polyacids, polyamines and dicyclocarbonates from vegetable oils and from glycerine derivatives. They were used for the synthesis of biobased polyurethanes, polyhydroxyurethanes and epoxy resins.
Key words: vegetable oils / thiol-ene / bio-based polymers / epoxy resins / polyurethanes / polyhydroxyurethanes
© John Libbey Eurotext 2013
In recent years, the sustainability is becoming increasingly important for the chemical industry; thus, the use of renewable resources has gained interest in polymer applications. Indeed, overall demand for chemical products will increase by 50% in volume by 2020 (Prudhon, 2010). Thus, American studies estimate that 90% of organic chemicals will come from renewable resources by 2090 (Eissen et al., 2002). However, it is not sufficient to synthesize exactly the same chemicals from renewable resources, even if they are harmful. Biobased chemicals could also be very dangerous. New processes have to be developed to replace hazardous reactives by harmless, biobased ones. Vegetable oils are extracted primarily from the seeds of oilseed plants. Their competitive cost, worldwide availability, and built-in functionality (ester functions and insaturations) make them attractive. The development of oleochemicals has been carried out from two distinct ways. The first one corresponds to the double-bond modification (Gunstone et al., 2001) of crude oils or fatty acid derivatives. The second one is the carboxylic acid group modification of vegetable oils (Corma et al., 2007). The chemical functionalizations of unsaturated oils to produce polyols have been widely developed to prepare new polyurethane structures, which depend on triglyceride and isocyanate reagents used (Zanetti-Ramos et al., 2006; Yeganeh et al., 2007; Guo et al., 2000). Demand for renewable resources is also increasing for polymers and composite applications. This demand is particularly strong for polyurethanes (PUs) and epoxy resins (ER) with a global production of respectively 14 Mt and 2 Mt per year (Shen et al., 2009). These polymers became among the most dynamic groups of polymers, exhibiting versatile properties suitable for use in practically all the fields of polymer applications – foams, elastomers, thermoplastics, thermosets, adhesives, coatings, sealants, fibers, and so on. In this context, our team synthesized new building blocks from vegetable oils in order to synthesize biobased PUs and ER materials. Experimental conditions and characterizations of these works were previously reported and scale-up was performed by Specific Polymers Company, Av. de l’Europe, 34830 Clapiers France.
Polyurethane precursors
PUs are obtained by the reaction of an oligomeric polyol (low molecular weight polymer with terminal hydroxyl groups) and a diisocyanate (or polyisocyanate). However, diisocyanates are not biobased and are generally very harmful reactants for human health. Thus, most used diisocyanates, methylene diphenyl diisocyanate (MDI) and toluene diisocyanate (TDI) are CMR products. Therefore the substitution of these compounds is crucial. To answer these questions, we proposed various solutions (figure 1). In a first approach, since polyols correspond to 70% w/w of PU we synthesized new biobased polyols from vegetable oils. In a second approach, we used a reaction which is currently gaining much attention as an alternative route for the synthesis of PUs: step-growth polyaddition of dicyclocarbonates and diamines (Whelan et al., 1963; Mikheev et al., 1983). This method is quite interesting since no hazardous isocyanates are used and dicyclocarbonate reactants can be obtained from renewable resources such as glycerin. Moreover, this route allows the synthesis of polyhydroxyurethanes (PHUs) with hydrogen bonds, which have higher chemical resistance and better hydrolysis behavior.
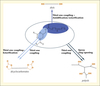 |
Figure 1. Synthetic ways from vegetable oils to polyurethanes precursors. |
Synthesis of di- and polyols by thiol ene coupling
On one hand, Soybean oil was reacted with mercaptoethanol in presence of an initiator (figure 2). The study of the addition of mercaptoethanol on oleic acid allowed defining the experimental conditions (Desroches et al., 2011): synthesis was done in mass, with a ratio of thiol/double bonds of 3:1, at 80°C in the presence of AIBN with a ratio initiator/double bonds of 0.1/1.
 |
Figure 2. Schematic reaction of mercaptoethanol grafting onto vegetable oil double bonds. |
Polyols were subsequently used to synthesize partially bio-based PUs materials. Formulations were performed with a MDI prepolymer with %NCO = 30.46 in the presence of functionalized triglycerides. Reaction mixtures exhibited a gel time of 170 min, monitored according to Winter Chambon criterion. The obtained materials had a Tg around 0°C (determined by Differential Scanning Calorimetry), a shore hardness D of 20, and a Young Modulus of 7 N/mm2 and tensile strengths at break of 1 MPa (Caillol et al., 2012). On the other hand, we have developed a synthetic strategy, which allows reaching a wide range of soft pseudo-telechelic diols from vegetable oils methyl esters. The soft segments of vegetable oils were comprised of either ester groups (one or two) or amide groups (one or two) with various spacer lengths between hydroxyl groups (figure 3). Thus, the synthetic pathway was the following: 1) transesterification with a diol or amidification with hydroxylamine reactant; 2) thiol-ene radical coupling in presence of mercaptoethanol (Desroches et al., 2012). Two main parameters seemed to govern the physical properties of these pseudo-telechelic diols: the nature of ester/amide group and the spacer length. These parameters positively or negatively influenced the hydrogen bonding between pseudo-telechelic diols and thus modified their physical properties. For instance, the glass transition temperature decreased when the spacer length increased, whereas the melting temperature of amide containing pseudo-telechelic diols was much higher than that of ester containing pseudo-telechelic diols.
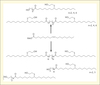 |
Figure 3. Ester/amide polyols from fatty acid esters. |
These pseudo-telechelic diols were reacted with MDI to elaborate PUs. It is particularly interesting to note that the thermostability of these PUs was lowered in the presence of amide groups. In the other hand, PUs with amide groups exhibited the highest glass transition temperatures (around 60°C), due to hydrogen bonding enhancement. Furthermore, chain length between functional groups – ester and amide – modified the rigidity of corresponding PUs. Finally, we demonstrated that amide groups influence the curing behavior through a catalytic effect onto the isocyanate-alcohol reaction (gel times around 40 min for diols with amide groups and around 200 min for diols with ester groups).
Polyols by epoxide ring opening
We also worked on epoxidized vegetable oils which are interesting industrial biobased resources. We thus synthesized biobased polyols by epoxide ring opening of epoxidized vegetable oils, with three different acids (figure 4): lactic and glycolic acids were selected since they are both biobased and present respectively a secondary and a primary hydroxyl group. Acetic acid, without hydroxyl group, was selected due to its low cost and widespread use in chemical industry. The polyol obtained from lactic acid is the most interesting in terms of renewable carbon content. It is noted that reactions occurred in mass, at relatively low temperatures, without initiator or purification, which meets the principles of green chemistry (Caillol et al., 2012a).
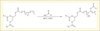 |
Figure 4. Ring opening of epoxidized vegetable oils by biobased acids. |
The three synthesized polyols led to materials with similar thermal and mechanical properties (Tg values around 50°C, tensile strengths at break > 20 MPa and Young Moduli > 900 N/mm2 at 23°C), except the gel time which strongly depended on the type of hydroxyl function of the precursor (from 370 min for glycolic acid polyol, which exhibits primary alcohols, to 690 min for acetic acid polyol, which bears only secondary alcohols). The tree PUs obtained from these polyols present a high content of renewable carbon, around 70%. The synthesis of PUs from vegetable oil based polyols was extensively reported in the literature. It is possible to compare PU from biobased polyols reacted with pure or modified MDI, with a NCO/OH ratio ranging from 1.00 to 1.05 (figure 5). Polyols from raw castor oil (Petrovic et al., 2008) (castor oil), or polymerized castor oil (Xu et al., 2008) (Es-pricin) led to low Tg PUs. Diesters synthesized by our team led also to low Tg PUs (DED). PUs from our monoesters diols (MED) showed Tg around 20°C. Thiol grafting onto vegetable oil allowed obtaining PUs with Tg ranging from 0°C to 20°C. The functionalization of vegetable oils by hydrogenation of epoxydized soybean oil (Petrovic et al., 2000) (Ep-H2), ozonolysis (Petrovic et al., 2005) (Dl-ozo), hydroformylation (Petrovic et al., 2008) (Dl-hydrof), and cyclocarbonate ring opening (Tamani et al., 2004) (Ep-carbonat) allowed to obtain Tg ranging from 20 to 40°C. Finally, to ensure high Tg (> 50°C), it is recommended to use the epoxy ring opening of vegetable oils, either by halogenated reactants (Petrovic et al., 2000) (Ep-HCl, Ep-HBr), or by acids (Miao et al., 2010) (Ep-lactiq, Ep-glycolic, Ep-acetic) or alcohols (Pechar et al., 2006) (Ep-MeOH). Amide diols synthesized by our team (MAD, DAD or MAT) led also to high Tg-PUs.
 |
Figure 5. Tg comparison of PUs obtained from functionalized vegetable oils and MDI-based isocyanate (blue for literature, green for our syntheses) (determined by DSC). |
Dicyclocarbonates for NIPUs
Isocyanate reactants are generally harmful for human health. Therefore the synthesis of PUs from step growth polyaddition of dicyclocarbonates and diamines should be favored. In that purpose, our team performed a new synthesis of 4-[(prop-2-en-1-yloxy)methyl]-1,3-dioxolan-2-one (AGC) by Williamson ether synthesis from 4-(hydroxymethyl)-1,3-dioxolan-2-one (glycerin carbonate) (Benyahya et al., 2011). Dicyclocarbonate was synthesized by UV thiol-ene coupling of AGC with a 2,20-oxydiethanethiol (figure 6). This photochemical thiol-ene reaction was carried out under air, with neither solvent nor photoinitiator.
 |
Figure 6. Dicyclocarbonate synthesis by thiol ene coupling on AGC. |
The synthesized dicyclocarbonate was used without purification to synthesize polyhydroxyurethanes without isocyanate by step growth polyaddition with 1,10-diaminodecane. The synthesized polyhydroxyurethane exhibited glass transition temperature of –31°C and a molecular weight of 9000 g/mol. This value was comparable to those reported in the literature. For instance, the polyaddition of 4,4’-[ethane-1,2-diylbis(sulfanediylbutane-4,1-diyl)]bis(1,3-dioxolan-2-one) led to a PHU with a yield of 67% and a molecular weight of 7500 g/mol (Tomita et al., 2001).
Hardeners for epoxy resins
The development of ecofriendly curing agents for epoxy resins is of great importance. Few solutions of nontoxic amine hardeners are reported in literature (Fenouillot et al., 2010). The diamines the most used in industry are methylenedianiline (MDA) and diaminodiphenylsulfone (DDS). However, DDS is toxic and MDA is a CMR chemical. Therefore their use is very harmful and should be avoided. Others amines are also used as epoxy resin hardeners, such as isophorone diamine and N-aminoethyl piperazine, but these amines remain toxic for human and environment. Besides amines, acid hardeners lead to interesting curing properties and some studies have proposed nontoxic or biobased acid hardeners for epoxy resins. Thus, a study reports the use of abietic acid and maleic acid to synthesize a diacid for epoxy curing (Wang et al., 2011). Acid functionalized lignin was also reported as epoxy hardener (Hiroko et al., 2009). Modified lignin with acid derivatives of mono and disaccharides were also used as hardeners (Hirose et al., 2003). Hardening of epoxy resins is performed at 130°C with reaction time between 6 and 10 hours. Moreover poly(styrene-co-acrylic acid) or poly(acrylic acid) was also used as acid hardener (Heba et al., 2003). The curing is rather slow, and uncompleted even at 100°C. Amino acids have also been studied, particularly lysine and tryptophan (Li et al., 2006). In both cases curing were performed above 150°C, even with a catalyst. All these works showed that only few acids and amine hardeners for epoxy have been synthesized in the past from renewable resources. Moreover, the applied methodologies lead generally to mono or difunctional precursors or imply multistep processes with low yields and formation of many by-products. Thus, we present the synthesis of polyacids based on unsaturated triglycerides thanks to the thiol-ene coupling and polyamine thanks to the amidification reaction (figure 7).
 |
Figure 7. Synthetic pathways from vegetable oils to epoxy resins precursors. |
Polyacids synthesized by thiol-ene coupling
New vegetable acids hardeners were prepared using thioglycolic acid by thiol-ene coupling (figure 8). The resulting polyacid exhibited a mean functionality of 3.3 measured by 1H NMR and titration.
 |
Figure 8. Synthesis of polyacids by thioglycolic acid grafting onto vegetable oils. |
The thermal crosslinking reaction between synthesized acids hardeners and commercial bisphenol A diglycidyl ether (BADGE) EPOTEC was studied. The DSC results showed that BADGE and synthesized fatty polyacid acid presented a Tg value of –18°C and –45°C, respectively, and the polymer obtained by curing of both reactants showed a Tg value of – 12°C. Synthesized polyacid could be used as hardener at low or high temperature for curing epoxy resins (table 1).
Gel time at different curing temperature of epoxy resins.
Curing times measured with the biobased polyacid hardener we synthesized are lower compared to other polyacid hardeners such as the poly(acrylic acid) polymers depicted by Heba et al. with 100 min at 100°C. The higher reactivity of our biobased hardener could be due to the activation of acid function by the presence of sulfur in the vicinity. Indeed, Miao et al. (Miao et al., 2010) have already evidenced the higher reactivity of acid hardener by the activating presence of oxygen in the vicinity. And sulfur and oxygen share some close properties in terms of inducting effect.
Polyamines synthesized by amidification reaction
Amine harderners were also synthesized by amidification of vegetable oils with diethylene triamine (figure 9). The product of the reaction is an amido-amine with an average functionality of 3.
 |
Figure 9. Amine hardener by vegetable oil amidification. |
The monoadduct was used as amine hardener with BADGE epoxy precursors. The resin obtained exhibited a Tg of 32°C. Other amines were designed from vegetable oils, by dimerization followed by amidification (Fomina, 2010), by thiol-ene coupling (Stemmelen et al., 2011), by nitrile synthesis (Dubois, Gillet, 2008) or by a 3 step reaction from epoxydized oil (Zao et al., 2008). But our method allows to synthesize fatty amido-amine in a one-step reaction.
Conclusion
We developed a real chemical toolbox based on thiol-ene coupling and amidification/esterification to synthesize a library of biobased building blocks with various functions and functionality from vegetable oils. The synthesized building blocks reported in this contribution are polyols, polyacids, polyamines and dicyclocarbonates from vegetable oils and from glycerine derivatives. They led to polymer synthesis such as polyurethanes, polyhydroxyurethanes and epoxy resins. These biobased building blocks led to polymers with various properties: low Tg polymers for coating or higher Tg polymers for composites.
Disclosure
Conflict of interest: none.
Acknowledgments
Authors thank Specific Polymers Company, Av. de l’Europe, 34830 Clapiers France for up scaling our syntheses.
References
- Benyahya S, Desroches M, Auvergne R, Carlotti S, Caillol S, Boutevin B. Synthesis of glycerine carbonate-based intermediates using thiol-ene chemistry and isocyanate free polyhydroxyurethanes therefrom. Polym Chem 2011; 2: 2661–2667. [CrossRef] [Google Scholar]
- Caillol S, Desroches M, Carlotti S, Auvergne R, Boutevin B. Synthesis of new polyurethanes from vegetable oil by thiol-ene coupling. Green Mater 2012; DOI: [10.1680/gmat.12.00001]. [Google Scholar]
- Caillol S, Desroches M, Boutevin G, Loubat C, Auvergne R, Boutevin B. Synthesis of new polyester polyols from epoxidized vegetable oils and biobased acids. Eur J Lipid Sci Technol 2012; DOI: [10.1002/ejlt.201200199]. [Google Scholar]
- Corma A, Iborra S, Velty A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev 2007; 107: 2411–2502. [CrossRef] [PubMed] [Google Scholar]
- Desroches M, Caillol S, Lapinte V, Auvergne R, Boutevin B. Synthesis of biobased polyols by thiol-ene coupling from vegetable oils. Macromolecules 2011; 44: 2489–2500. [CrossRef] [Google Scholar]
- Desroches M, Caillol S, Auvergne R, Boutevin B. Synthesis of pseudo-telechelic diols by trans-esterification and thiol-ene coupling. Eur J Lipid Sci Technol 2012; 114: 84–91. [CrossRef] [Google Scholar]
- Desroches M, Caillol S, Auvergne R, Boutevin B, David G. Biobased cross-linked polyurethanes obtained from ester/amide pseudo-diols of fatty acid derivatives synthesized by thiol-ene coupling. Polym Chem 2012; 2: 450–457. [CrossRef] [Google Scholar]
- Dubois JL, Gillet JP. Coproduction of cyclic carbonates and of nitriles and/or of fatty amines. Arkema Patent WO2008145941A2, 2008. [Google Scholar]
- Eissen M, Metzger J, Schmidt E, Schneidewind U. 10 year after Rio – Concepts on the contribution of chemistry to a sustainable development. Angew Chem Int Ed 2002; 41: 414–436. [CrossRef] [Google Scholar]
- Fenouillot F, Rousseau A, Colomines G, Saint-Loup R, Pascault JP. Polymers from renewable 1,4:3,6-dianhydrohexitols (isosorbide, isomannide and isoidide): A review. Prog Polym Sci 2010; 35: 578–622. [CrossRef] [Google Scholar]
- Fomina EV. Synthesis and properties of new polyfunctional curing agents for epoxy resins based on dimerized fatty acids. Polym Sci 2010; 3: 87–91. [Google Scholar]
- Gunstone FD. Chemical reactions of fatty acids with special reference to the carboxyl group. Eur J Lipid Sci Technol 2001; 103: 307–314. [CrossRef] [Google Scholar]
- Guo A, Javni I, Petrovic Z. Rigid polyurethane foams based on soybean oil. J. Appl. Polym. Sci. 2000; 77 : 467–473. [CrossRef] [Google Scholar]
- Heba F, Mouzali M, Abadie JM. Effect of the crosslinking degree on curing kinetics of an epoxy-acid copolymer system. J Appl Polym Sci 2003; 90: 2834–2839. [CrossRef] [Google Scholar]
- Hiroko W, Shinetsu F, Taro F, Akinori M, Ari K, Yumiko O. Adsorbent and method of manufacturing the same. Toshiba. Patent JP2009034634, 2009. [Google Scholar]
- Hirose S, Hatakeyama T, Hatakeyama H. Synthesis and thermal properties of epoxy resins from ester-carboxylic acid derivative of alcoholysis lignin. Macromolecular Symp 2003; 197: 157–169. [CrossRef] [Google Scholar]
- Li Y, Xiao F, Moon KS, Wong CP. Novel curing agent for lead-free electronics: amino acids. J Polym Sci Part A: Polym Chem 2006; 44: 1020–1027. [CrossRef] [Google Scholar]
- Miao S, Zhang S, Su Z, Wang P. A novel vegetable oil-lactate hybrid monomer for synthesis of high-Tg polyurethanes. J Polym Sci Part A: Polym Chem 2010; 48: 243–250. [CrossRef] [Google Scholar]
- Mikheev W, Svetlakov NV, Sysoev VA and Gumerova RK, Zh Org Khim 1983; 19: 498–501. [Google Scholar]
- Pechar TW, Sohn S, Wilkes GL, et al. Characterization and comparison of polyurethane networks prepared using soybean-based polyols with varying hydroxyl content and their blends with petroleum-based polyols. J Appl Polym Sci 2006; 101: 1432–1443. [CrossRef] [Google Scholar]
- Petrovic ZS, Guo A, Zhang W. Structure and properties of polyurethanes based on halogenated and nonhalogenated soy-polyols. J Appl Polym Sci 2000; 38: 4062–4069. [CrossRef] [Google Scholar]
- Petrovic ZS, Zhang W, Javni I. Structure and Properties of Polyurethanes Prepared from Triglyceride Polyols by Ozonolysis. Biomarcomolecules 2005; 6: 713–719. [CrossRef] [PubMed] [Google Scholar]
- Petrović ZS, Cvetković I, Hong D, et al. Polyester polyols and polyurethanes from ricinoleic acid. J Appl Polym Sci 2008; 108: 1184–1190. [CrossRef] [Google Scholar]
- Petrovic ZS, Guo A, Javni I, Cvetkovic I, Hong DP. Polyurethane networks from polyols obtained by hydroformylation of soybean oil. Polym Int 2008; 57: 275–281. [CrossRef] [Google Scholar]
- Prudhon P. Industrie chimique et le grenelle de l’environnement. Union des industries chimiques 2010. [Google Scholar]
- Shen L, Haufe J, Patel MK. Product overview and market projection of emerging biobased plastics, Utrecht University commissioned by European Polysaccharide network of excellence and European bioplastics 2009. [Google Scholar]
- Stemmelen M, Pessel F, Lapinte V, Caillol S, Habas JP, Robin JJ. A fully biobased epoxy resin from vegetable oils: From the synthesis of the precursors by thiol-ene reaction to the study of the final material. J Polym Sci Part A: Polym Chem 2011; 49: 2434–2444. [CrossRef] [Google Scholar]
- Tamami B, Sohn S, Wilkes GL. Incorporation of carbon dioxide into soybean oil and subsequent preparation and studies of nonisocyanate polyurethane networks. J Appl Polym Sci 2004; 92: 883–891. [CrossRef] [Google Scholar]
- Tomita H, Sanda F, Endo T. Polyaddition of bis(seven-membered cyclic carbonate) with diamines: A novel and efficient synthetic method for polyhydroxyurethanes. J Polym Sci Part A: Polym Chem 2001; 39: 4091–4100. [CrossRef] [Google Scholar]
- Wang H, Wang H, Zhou G. Synthesis of rosin-based imidoamine-type curing agents and curing behavior with epoxy resin. Polym Int 2011; 60: 557–563. [CrossRef] [Google Scholar]
- Whelan Jr JM, Cotter RJ. Multiple cyclic carbonate polymers. US Paten t3072613, 1963. [Google Scholar]
- Xu Y, Petrovic Z, Das S, Wilkes GL. Morphology and properties of thermoplastic polyurethanes with dangling chains in ricinoleate-based soft segments. Polymer 2008; 49 : 4248–4258. [CrossRef] [Google Scholar]
- Yeganeh H, Hojati-Talemi P. Polym. Preparation and properties of novel biodegradable polyurethane networks based on castor oil and poly(ethylene glycol). Degrad Stab 2007 ; 92: 480–489. [CrossRef] [Google Scholar]
- Zao HP, Zhang JF, Sun XS, Hua DH. Syntheses and properties of cross-linked polymers from functionalized triglycerides. J Appl Polym Sci 2008; 110: 647–656. [CrossRef] [Google Scholar]
- Zanetti-Ramos BG, Lemos-Senna E, Soldi V, Borsali R, Cloutet E, Cramail H. Polyurethane nanoparticles from a natural polyol via miniemulsion technique. Polymer 2006; 47: 8080–8087. [CrossRef] [Google Scholar]
To cite this article: Desroches M, Auvergne R, Boutevin B, Caillol S. Synthesis of bio-based building blocks from vegetable oils: a platform chemicals approach. OCL 2013; 20(1): 16–22. doi : 10.1051/ocl.2012.0489
All Tables
All Figures
 |
Figure 1. Synthetic ways from vegetable oils to polyurethanes precursors. |
| In the text | |
 |
Figure 2. Schematic reaction of mercaptoethanol grafting onto vegetable oil double bonds. |
| In the text | |
 |
Figure 3. Ester/amide polyols from fatty acid esters. |
| In the text | |
 |
Figure 4. Ring opening of epoxidized vegetable oils by biobased acids. |
| In the text | |
 |
Figure 5. Tg comparison of PUs obtained from functionalized vegetable oils and MDI-based isocyanate (blue for literature, green for our syntheses) (determined by DSC). |
| In the text | |
 |
Figure 6. Dicyclocarbonate synthesis by thiol ene coupling on AGC. |
| In the text | |
 |
Figure 7. Synthetic pathways from vegetable oils to epoxy resins precursors. |
| In the text | |
 |
Figure 8. Synthesis of polyacids by thioglycolic acid grafting onto vegetable oils. |
| In the text | |
 |
Figure 9. Amine hardener by vegetable oil amidification. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.




